| Sep 11, 2012 |
Multiple networks of defects promote superconductivity
|
|
(Nanowerk News) Electronic devices such as integrated circuits work because of the defects in the underlying semiconductor material, namely silicon. Defects also control the electrical properties of many other important materials, including the ability of oxides of copper to superconduct at high temperatures, that is to conduct electricity with no resistance. This is a very useful property when we wish to design electromagnets, for example for hospital magnetic resonance imaging devices, which are completely stable and do not heat up upon use. For the copper oxides the organization of the defects strongly affects the superconductivity, most notably the temperatures to which the superconductivity survives.
|
|
A collaboration between the University of Rome and the London Centre for Nanotechnology (LCN) now reports, in an article published in the Proceedings of the (US) National Academy of Sciences ("Optimum inhomogeneity of local lattice distortions in La2CuO4+y"), that it is the organization of two - not one - types of defect, that determines the superconductivity of a particularly simple “parent” oxide of copper where additional oxygen atoms are introduced to induce superconductivity. The first defects are the additional oxygen atoms while the second are deviations of atoms from where they would have been in the “parent” material.
|
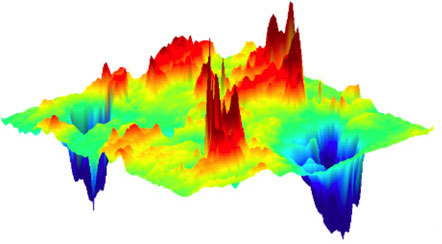 |
| Map of anticorrelated defects in optimized copper oxide superconductor. Red peaks correspond to the best organized distortions of the underlying atomic lattice while blue valleys represent oxygen defect order.
|
|
Earlier work by the Rome/LCN group has shown that the oxygen defect order can be highly inhomogeneous, even in optimal superconducting samples, which raises the question of the nature of the sample regions where the order does not exist but which nonetheless form the “glue” binding the ordered regions together. The collaboration has now used X-ray microscopy, carried out at the European Synchrotron Radiation Facility in Grenoble, France, to show that the glue regions contain ordered defects of the second kind and that connectivity of the ordered defects is most pronounced for the best superconducting samples. The latter exist in droplets anticorrelated with the ordered excess oxygen atoms whose connectivity is also most pronounced for the best superconducting samples.
|
|
There are therefore two – not only the one found previously - networks of ordered defects which must be tuned to achieve optimal superconductivity. For a given concentration of excess oxygen atoms, the highest transition temperature is obtained when both the ordered oxygen and lattice defects form maximally connected yet anticorrelated patterns, as opposed to appearing in isolated spots.
|
|
Prof. Bianconi of the University of Rome, noted that “The results further reinforce the idea that the remarkable superconductivity of the copper oxides cannot be understood in terms of the standard approach to superconductivity from the 1950’s where we think of electron pairing in a homogeneous medium as for ordinary solids”. Prof. Aeppli, Director of the London Centre for Nanotechnology, added that “The experiments also suggest that engineered heterogeneity, along the lines of what occurs with the heat treatment of the rather simple oxide of copper which we have studied, may lead to even more remarkable superconductors in the future.”
|

