| Sep 26, 2012 |
From sunlight to hydrogen with a new hybrid material
|
|
(Nanowerk News) The ideal solution for making storable solar energy would be to directly convert sunlight into hydrogen fuel. In order to achieve this, it is essential to development new kinds of semiconductor materials that are capable of collecting light when dipped into water. These semiconductors would then utilize the light energy to produce hydrogen - all of which is a formidable technological challenge. As part of the BMBF excellence cluster project Light2Hydrogen, Helmholtz Zentrum Berlin (HZB) scientists – along with their colleagues from partnering research institutes – have prepared and characterized a new type of hybrid material capable of doing just that. The photoelectrochemical reaction occurs on photocatalytic active carbon nitride films (called polymeric carbon nitride) which are deposited onto semiconducting substrates like chalcopyrite or silicon.
|
|
For the first time, the scientists have demonstrated that polymeric carbon nitride films coated on p-type chalcopyrite and silicon, respectively, can be successfully applied as new photoelectrochemical composite photocathodes for light induced hydrogen production ("Metal-Free Photocatalytic Graphitic Carbon Nitride on p-Type Chalcopyrite as a Composite Photocathode for Light-Induced Hydrogen Evolution").
|
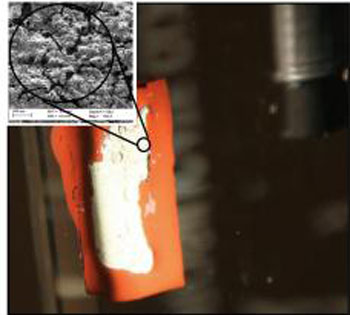 |
| Solar hydrogen evolution by graphitic carbon nitride/p-type chalcopyrite thin film photo-cathode: It is shown that graphitic carbon nitride films coated on p-type CuGaSe2 thin films can be successfully applied as new photoelectrochemical composite photo-cathode for light induced hydrogen evolution.
|
|
Solar energy can be used to split water into oxygen and hydrogen. The resulting hydrogen is a fuel capable of being compressed or chemically converted, and subsequently, stored. To date, there are no well-engineered material systems which exist for hydrogen production that use artificial photosynthetic systems. Water-dipped semiconductors require the ability to absorb visible sun light for an efficient charge-carrier generation and transport to the semiconductor surface. Of course, the ideal candidate semiconductors are silicon or chal-copyrite, those used in photovoltaics. However, if dipped in water, silicon and chalcopyrite instantly start to corrode, rendering them ineffective. This is precisely the reason why scientists have been searching for other types of semiconductors or other appropriate photocatalytic materials like polymeric carbon nitride.
|
|
Until now, this substance was only utilized in a powdery form. In the frame of the "Light2Hydrogen" project, scientists have at last succeeded in depositing films of polymeric carbon nitride onto chalcopyrite and silicon. The protocols for doing so are already well established at HZB, as they are used, for example in thin film solar cell research at the Centre. "The new composite material has been used at low pH values – in other words, under acidic conditions, for the application as a photoelectrode," explains PD Dr. Thomas Schedel-Niedrig, the scientist in charge of the project at HZB. The composite material turned out to be stable and capable of generating large quantities of hydrogen under illumination with visible light. "Through this hybrid connection of polymeric carbon nitride with either chalcopyrite or silicon, we implemented an additional electric field at the composite interface, which enhances performance."
|
|
Needless to say, the scientists were not content enough with the initial results of their discovery. So they sought to understand the details of how polymeric carbon nitride binds to the chalcopyrite or silicon surface, and how to decrease the polymeric carbon nitride film thickness by metal atom incorporation in order to enhance the electric conductivity. With this knowledge, the scientist could optimize the photoelectrochemical.
|
|
“We conducted relevant tests using water vapor at an experimental endstation at the Fritz-Haber-Institute using the X-rays from the synchrotron radiation source BESSY II”, says Schedel-Niedrig. "There, we were able to spectroscopically analyze the surface components under ambient conditions, i.e. “in-situ”, with a high-degree of accuracy in order to specifically modify them to suit our needs.”
|
|
This is necessary to increase the hydrogen yield and to ensure that the chemical reaction takes place not only in sulphuric acid but also, later on in water. "If we want to do our part to help create new energy source concepts from our fundamental scientific research, we have to continue to develop the protocols in such a way that, later on, they can be ap-plied to an industrial application," explains the HZB scientist.
|
|
The prospects are promising. In fact, HZB has just been accepted as a partner within the DFG Priority Programme “Renewable Fuels Produced Through Light-Driven Water Splitting: Clarification of the Elemental Processes Involved and Prospects for Implementation in Technological Concepts” (SPP 1613). Together with colleagues of the Technical University Berlin and the Fritz-Haber-Institute of the Max Planck Society, the HZB scientists will be working on making sunlight a viable source of hydrogen fuel production using chalcopyrite related photocathodes, and additionally, for the oxygen evolution reaction using tantalum oxinitride-based photoanodes.
|

