| Nov 27, 2012 |
Size matters when reducing nickel oxide nanoparticles
|
|
(Nanowerk News) New research finds that size plays a major role in how nanoscale nickel oxide (NiO) shells behave when being reduced to solid nickel nanoparticles.
|
|
“This advances our fundamental understanding of how the structures of nanoparticles can be changed through chemical reactions, which has potential applications in nanofabrication and catalysis,” says Joe Tracy, a materials scientist at NC State and co-author of a paper on the work ("Nanostructural transformations during the reduction of hollow and porous nickel oxide nanoparticles ").
|
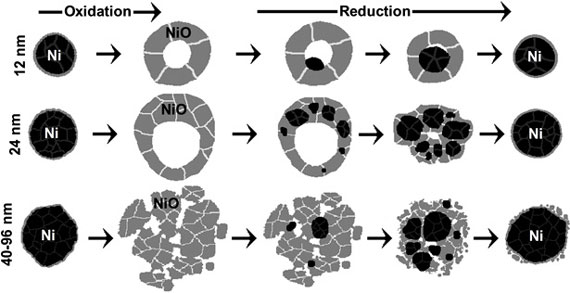 |
| The number and location of nucleation sites of nickel metal within nickel oxide nanoparticles during their reduction with hydrogen strongly depend on the nanoparticle size.
|
|
The researchers began by exposing nickel nanoparticles to air at 500 degrees Celsius in order to create NiO shells. This process is called oxidation. The smaller shells, 12 to 24 nanometers (nm) in diameter, are hollow, with the shell surrounding a single cavity. Larger shells, 40 to 96 nm in diameter, appear to have larger pores, and possibly contain multiple cavities.
|
|
The researchers then placed the shells in a hydrogen gas environment at 350 degrees C. This process, called reduction, turns the NiO shells back into solid nickel nanoparticles.
|
|
What they found was that the size of the NiO shells dramatically affects the way that the reduction process manifests itself.
|
|
The smallest shells the researchers looked at, 12 nm in diameter, formed a single nucleation site of pure nickel, which then expanded to replace all of the NiO. Larger shells, 24 nm in diameter, responded differently – forming multiple nucleation sites in an approximate ring shape around the shell. These nucleation sites then grew and merged into a single nickel nanoparticle. The largest shells they looked at, 96 nm in diameter, looked more different still, with multiple nucleation sites forming throughout the NiO.
|
|
“The size of the nanoparticles before oxidation determines both the structure of the NiO nanoparticles and the pattern of the nucleation sites of nickel metal during reduction,” says John Medford, an undergrad at NC State and lead author of the paper.
|

