| Feb 22, 2013 |
Researchers demonstrate fullerene crystals with bimodal pore architectures
|
|
(Nanowerk News) A research group headed by MANA Scientist Dr. Lok Kumar Shrestha of the Supermolecules Unit, in collaboration with MANA Independent Scientist Dr. Yusuke Yamauchi for the first time demonstrated template-free novel mesoporous carbon material: fullerene (C60) crystals with bimodal pore architectures and having highly crystallized framework. Experiments have proven that this novel meso- and macroporous material show better electrochemical performance compared to pristine C60 due to higher electrochemically active surface areas ("Fullerene Crystals with Bimodal Pore Architectures Consisting of Macropores and Mesopores").
|
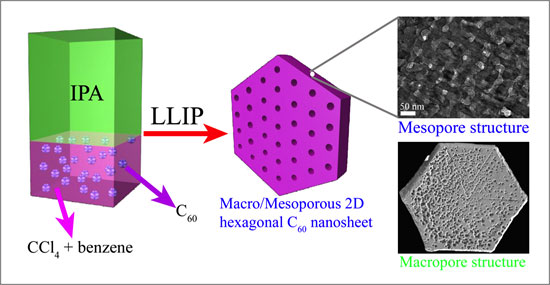 |
| Synthetic route of producing mesoporous crystalline fullerene using a liquid-liquid
|
|
In this research, novel fullerene crystals with bimodal mesoporous and macroporous structures composed of a highly crystallized framework has prepared by using a liquid-liquid interfacial precipitation (LLIP) method involving the interface between isopropyl alcohol (IPA) and a saturated solution of C60 in a mixture of benzene and carbon tetrachloride (CCl4). The resulting mesoporous C60 exhibits two-dimensional (2D) hexagonal plate morphology.
|
|
Porosity and electrochemically active surface area could be flexibly controlled by increasing the mixing fraction of CCl4 and benzene. The synergistic effect of mixing solvents (CCl4 and benzene) is mainly responsible for the formation of such porous structure. Otherwise, in an individual IPA/CCl4 and IPA/benzene system, 2D plate like and 1D nanowhiskers morphology without any pores are observed. In solution-based crystallization (LLIP method), solvent molecule gets entrapped during crystallization, which upon slow release/or evaporation creates a porous structure. It is expected that this methodological innovation will be a milestone for the production of highly crystalline carbon-based materials offering better performance in catalytic, electrochemical, and sensing properties.
|

