| May 02, 2014 |
New atom-scale knowledge on the function of biological photosensors
|
|
(Nanowerk News) The research groups of Janne Ihalainen (University of Jyväskylä) and Sebastian Westenhoff (University of Gothenburg) have clarified how the atom structure of bacterial red light photosensors changes when sensing light. The research ("Signal amplification and transduction in phytochrome photosensors") reveals structural changes in phytochrome protein when illuminated.
|
 |
| A familiar phenomenon: plants turn toward light. Plants sense light with their photosensors, from which phytochromes are the proteins to sense red light.
|
|
“The results are a unique demonstration of proteins’ ability to structural changes in different phases of their operation. This helps to understand how the biological photosensors function. The modelling and utilisation of protein for other applications becomes much easier when the protein structures, their changes and the speed of change are known,” says Professor Ihalainen.
|
|
The function of few biological photosensors are already utilised in other fields of science, especially in neurosciences. By utilising reactions that are controlled by light, it is possible to achieve new breakthroughs in the cell biological research and, for example, in medical applications such as in phototherapy and in molecular diagnostics.
|
|
Organisms use photosensor proteins to sense light on different wavelengths. For example, mammals have rhodopsin proteins in their eyes. Phytochromes, one of the photosensor proteins of plants, fungi and bacteria, are sensitive to red light. The function of these photosensors was known already in 1970s and 1980s, but their molecular-level operating mechanisms are still unknown.
|
|
A pioneering research method
|
|
Time-resolved wide-angle X-ray scattering was used to study structural changes of this rather large protein complex in a solution form. The technique, TR-WAXS, is relatively new and in this study a successful combination of the experimental data with the molecular dynamic simulations enabled to track the detailed structural changes of the protein.
|
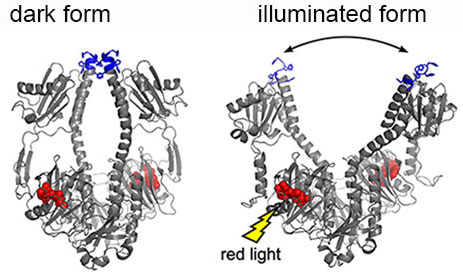 |
| The crystal structure of bacterial phytochrome changes when illuminated.
|
|
“We hope that other groups using TR-WAXS would test similar data-analysis method as well.” Ihalainen says.
|
|
The light sensitive phytochrome structures were clarified both in a crystal form and in a solution. From the crystal structures, it is possible to see that small movement of individual atoms (scale of 0.1 – 0.2 nm) caused by the absorption of light is amplified to large structural changes (3 nanometres) in the whole protein complex. This amplification mechanism enables the light induced signal transmission from one protein to another very quickly and with precise replication accuracy. In turn, this signal transmission process initiates cellular-level changes in the organism.
|
|
The project “New strategies for detection conformational changes of proteins in real time” is funded by the Academy of Finland.
|


