| May 07, 2014 |
Catalysis with gold-tipped CdSe nanorod clusters
|
|
(Nanowerk News) Clusters of gold (Au)-tipped CdSe nanorods were synthesized by researchers in the Center for Nanoscale Materials Nanophotonics Group through nanorod polymerization driven by the controlled welding of the Au tips. The Au-tipped CdSe nanorods were found to significantly increase the efficiency for charge separation and collection of energetic electrons in the Au nanoparticles, leading to an exceptional improvement in photocatalyzing multiple-electron reduction (MER) reactions, even in aerobic aqueous solutions at room temperature.
|
|
The results ("Promoting photocatalytic multiple-electron reduction in aerobic solutions using Au-tipped CdSe nanorod clusters") represent the first use of hybrid metal-semiconductor nanomaterials for efficient photocatalysis involving multiple-charge reactions.
|
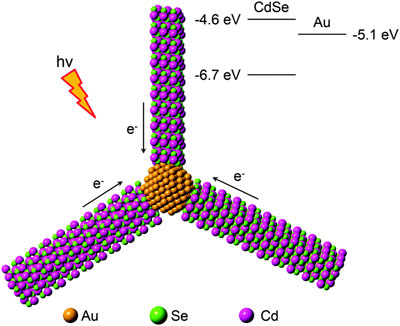 |
| Figure 1. Hybrid metal-semiconductor nanorod cluster that can promote photocatalytic multiple-electron reduction reactions. The energy diagram highlights the band edges of CdSe and the Fermi level of Au.
|
|
Figure 1 shows the proposed nanostructure, consisting of multiple CdSe nanorods that are linked to each other by an Au nanoparticle at their ends. The CdSe nanorods serve as antennae for efficient collection of photons to create energetic charge-separated states in the CdSe nanorods, while the Au nanoparticle serves as an electron reservoir for collecting energetic electrons generated from the charge-separated states. Under photoillumination, charge-separated states in each CdSe nanorod form and the resulting energetic electrons are transferred to the Au nanoparticle.
|
|
As a result, the number and density of energetic electrons accumulated in the Au nanoparticle is higher than that formed in the individual Au-tipped CdSe nanorod within the same time. The synthesis is realized through polymerization of the Au single-tipped CdSe nanorods by taking advantage of spontaneous welding of the Au tips under appropriate conditions (see Figure 2).
|
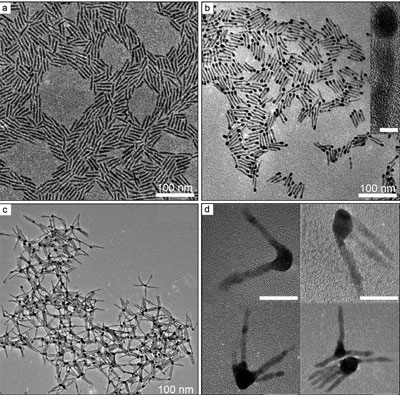 |
| Figure 2. TEM images of (a) the synthesized CdSe nanorods, (b) Au-tipped CdSe nanorods and each nanorod with a single Au nanocrystal, and (c) clusters of Au-tipped CdSe nanorods. (d) Enlarged TEM images of individual clusters of Au-tipped CdSe nanorods shown in (c), highlighting the different number (n) of CdSe nanorods in each cluster. Scale bars in the inset of (b) and the frames of (d) correspond to 5 and 20 nm, respectively.
|
|
To evaluate the performance in photocatalytic MER reactions, methylene blue (MB) was selected as a model redox indicator in aqueous solution. The MB molecules were photocatalytically reduced with the assistance of the Au-tipped CdSe nanorods under an ambient environment (i.e., the solution is saturated with air). The MB molecules were completely reduced within only 1 hour and MER first-order reaction kinetics were determined.
|


