| May 15, 2014 |
A new way to identify the atomic origin of molecular valence electrons
|
|
(Nanowerk News) We often classify the electrons in an atom as being either ‘core’ electrons – those closest to the nucleus – or ‘valence’ electrons, those involved in chemical bonding. Indeed, if the atom is part of a molecule, these electrons connect it to its neighbours. In this case, these valence electrons are delocalized over the entire molecule, making it difficult to identify which atom they really belong to. Now, a team of French, Swedish, and American scientists has managed to trace the origins of valence electrons that are ejected when x-rays strike a molecule. They were able to achieve this result thanks to the sensitivity and accuracy of the measuring instruments available at the PLEIADES beamline at the Synchrotron SOLEIL laboratory.
|
|
Their work appeares in Nature Communications ("Site-selective photoemission from delocalized valence shells induced by molecular rotation").
|
 |
| Illustration of the effect of electron ejection according to the atom of origin: the electronic emission spectrum for a lightweight atom is much wider and is shifted (green) with respect to that of a heavier atom (purple).
|
|
In practice, this new identification technique works best for molecules made up of both heavy and light atoms. The scientists used SOLEIL’s synchrotron radiation to shoot x-ray photons on a hydrogen chloride (HCl) molecule. These photons have enough energy to eject valence electrons from the molecule. How could one trace these electrons back to their atom of origin?
|
|
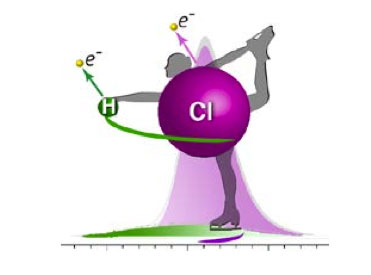
The ingenious way discovered by the researchers is to use the rotation of the molecule. When a photon strikes the molecule, an electron is ejected, and this affects the molecular rotation. If the atom is heavy, the ‘perturbation’ of the molecular rotation will be small. If the atom is light (like hydrogen, which has just one proton and one electron), the rotational movement imparted by the ejection of the electron will be much larger. The scientists detect the ejected electrons and record their energy distribution in the form of spectra. The observed spectral lines exhibit a horizontal shift compared to the expected values. It was also found that the spectrum associated to a lightweight atom is broader in energy than for a heavy one.
|
|
This phenomenon has never been observed before. X-ray photons were needed in order to disentangle the various contributions because they are much closer (as represented by the green and purple curves in the figure), and therefore more difficult to distinguish, than those observed for core electrons. However, there is a larger transfer of angular momentum at the time of the electron ejection, which makes the detection more sensitive.
|
|
Responsible of these promising results is also the top-level instrumentation available at the PLEIADES beamline. The method is possible to adapt to other molecules which have an atomic mass ‘imbalance’. Research along these lines could provide insight into some major scientific issues, since valence electrons often define the physical properties of a material or the chemical reactivity of a molecule. By revealing the atomic composition of the molecular orbitals, this new method could lead to a better understanding of electric and magnetic properties of matter. Such knowledge could hold the key to research on any new material.
|

