| Posted: Oct 30, 2009 | |
Dissecting the nanoworld: The atomic force microscope nanoscalpel |
|
| (Nanowerk Spotlight) Traditional techniques in cell biology involve chemical or pharmaceutical treatments of entire cells; however, in many cases it would be advantageous to target a single organelle or other structure within a cell without damaging overall cell structure. If scientists could inject a drug into a chosen organelle within the cell, or even destroy, extract or isolate the whole organelle without significantly harming the cell itself, new insight could be gained into the inner workings of the cell. | |
| In recent years, techniques have been developed which allow the manipulation of the individual nanoscale structures within biological cells. This manipulation, or “nanosurgery”, has the potential to provide new insight into the internal structure and dynamics of cells. Nanosurgical methods have been developed to target the cell’s internal organelles, the cell membrane, and the structural protein filaments within the cell (known as the “cytoskeleton”). | |
| Among the nanomanipulation techniques which exist, the atomic force microscope (AFM) is capable of imaging and working with extremely small structures with very high precision. | |
| Invented in the 1980s, AFM allows the imaging of biological cells with extremely high resolution, comparable to that of electron microscopes. However, unlike the electron microscope, which requires high vacuum conditions, the AFM can operate in ambient air, or even in fluid environments supporting living cells. This makes it an ideal tool for biological imaging, and the AFM probe can also be used as a tool to manipulate objects on the nanoscale. | |
| The AFM provides high resolution imaging as well as sub-nanometre control of the probe positioning, allowing a specific site or structure within a cell to be targeted with extremely high accuracy. However, the pyramid-like shape of the AFM tip reduces the precision of manipulation below the surface of a cell. | |
 |
|
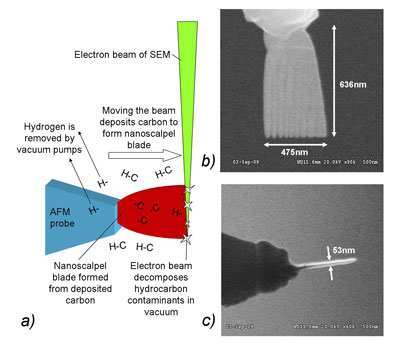
| Fig 1: a) Process of fabrication of nanoscalpel blade by electron-beam induced deposition (EBID). b) and c) Nanoscalpel blades imaged from the side (b) and edge (c). The amorphous carbon blade is robust and has a high-aspect ratio, making it ideal for nanosurgery. | |
| In a recent paper in Nanotechnology ("An atomic force microscope nanoscalpel for nanolithography and biological applications"), we described how our group at the Department of Physics of the University of Bath have developed new modified AFM probes designed for the cutting and manipulation of structures at extremely small scales. | |
| At the apex of this probe is a blade-like structure composed of amorphous carbon, a material far more robust than the crystalline silicon which makes up standard AFM probes. Using an AFM system this probe can be used for solid-state nanofabrication, and for the manipulation of biological cells. The nanoscalpel blades are fabricated on AFM probes using electron beam induced deposition (EBID). In this process, hydrocarbon vapour present in the vacuum chamber of a scanning electron microscope (SEM) is decomposed by the electron beam and the lighter, volatile hydrogen-rich fraction of the molecules is extracted by the vacuum system while the heavier carbon is deposited onto surfaces near the electron beam. The deposited carbon can be used to create nanoscale structures, and by moving the electron beam it is possible to fabricate 3-D nanostructures such as the nanoscalpel blades. | |
 |
|
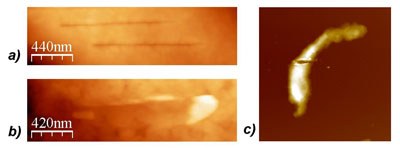
| Fig 2: a) Fine nanosurgical incisions on the surface of a fixed megakaryocyte cell, performed using a nanoscalpel probe and imaged with AFM. b) Incision made using unmodified AFM probe. This incision is comparatively wide, demonstrating that the nanoscalpel is able to make much finer incisions. c) A fixed smooth muscle cell which has been cut through completely by applying a large force to the nanoscalpel, isolating the two halves of the cell. (Reprinted with permission from IOP Publishing Ltd.) | |
| In solid-state nanofabrication, our group uses the nanoscalpel to cut 20-50nm wide gaps into metal films, forming a pair of contacts for the investigation of the electronic properties of nanoparticles and other nano-objects. Such gaps are difficult to fabricate reliably using conventional nanofabrication techniques but can be quickly and reproducibly created using a nanoscalpel. | |
| In biology, we have shown that the nanoscalpel can make fine incisions on the surface of fixed cells, and we believe that it has the potential to be used for the isolation of parts of the cell and the cutting or extraction of individual organelles or protein filaments, both in living and fixed cells. There is also the potential to “dissect” individual cells, exposing their internal structure for imaging in situ. This makes the nanoscalpel the smallest surgical implement in existence, capable of cutting structures much smaller than a single cell. | |
| The nanoscalpel offers several significant advantages over other existing nanomanipulation techniques, such as laser microscalpels. The use of an AFM system allows the nanoscalpel to be positioned with sub-nanometre accuracy, and since the AFM is also capable of high precision force measurement, both the position of the scalpel and the force applied to the cell can be accurately determined. With this data, it is possible to distinguish and determine the elastic and inelastic deformation of the cell by the scalpel. | |
 |
|
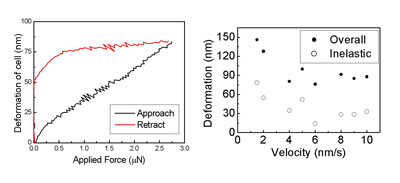
| Fig 3: Measurement of the force applied to the AFM probe allows the determination of the overall, elastic and inelastic deformation of the cell by the nanoscalpel. This allows mechanical properties of the cell to be investigated; in this case, viscoplastic-like behaviour is observed as the deformation varies with the velocity at which the nanoscalpel is moved towards the cell surface. (Reprinted with permission from IOP Publishing Ltd.) | |
| Not only does this provide control of the depth of penetration of the scalpel into the cell, but also has the potential to be used to determine the local elasticity and other mechanical properties of the cell. We have been able to show that fixed cells are deformed in a "viscoplastic" manner by the scalpel; this means that the cell behaves as a rigid object if force is applied rapidly, with greater deformation occurring if force is applied more slowly. It is known that elasticity and other mechanical properties change significantly in cancer cells, and the nanoscalpel could be used to investigate these changes, even at significant depths below the cell surface. There is even the potential, therefore, for the scalpel to be used as a diagnostic tool for cancer and other diseases. | |
| All this means that the nanoscalpel and other nanotools have the potential to significantly expand the range of nanosurgical techniques which can be performed using AFM, and would be useful in many fields including cell biology, pharmacology and medicine. | |
| By J.D. Beard. James is a PhD student in the Department of Physics at the University of Bath and first author of the above-mentioned paper in Nanotechnology. Copyright Nanowerk LLC | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
