| Posted: Mar 08, 2011 | |
Impact of nanoscale topography on genomics and proteomics of adherent bacteria |
|
| (Nanowerk Spotlight) One of the main basic bacterial survival strategies is the colonization of a surface and the consequent growth as biofilm community, which is embedded in a gel-like polysaccharide matrix (also known as exopolysaccharides matrix or EPS). In spite of its swimming/planktonic counterpart, such sessile adherent bacterial population represents an excellent life-support system. | |
| A biofilm like bacteria community is in fact highly resistant to almost any classical bactericidal and bacteriostatic tools, ranging from broad-spectrum antibiotics to UV-rays, disinfectant, heat, and so on. For this reason, all medical implant devices (i.e. intravascular catheters, pacemakers, ventricular devices, voice prostheses, and dental and orthopedic implants) may be subjected to severe, incurable and repetitive infection diseases, whose only way of recovery is the physical removal of such implants. | |
| Thus, the design of biomaterials with active antibacterial and self-cleaning properties represents a good opportunity for solving the biofilm associated infections. One of the main goal is avoiding one of the first necessary steps required for the biofilm growth, namely the bacterial adhesion onto the target surface. | |
| In such a context, all the scientific efforts in the last decade were mainly addressed to understand the physicochemical theories and characteristics of bacteria-surface interaction. In particular, different materials with different surface modifications and topographies (e.g., substrates with flat, rough, nanostructured surfaces, etc.) were investigated in order to understand which one of these represent the best bacteria-repulsive device. | |
| However, although all such noteworthy physical approaches revealed many and useful characteristics of the bacteria-surface interaction event, none of them explained what are the "biochemical" bases of such interactions. | |
| In our approach, reported in the February 23, 2011, online edition of ACS Nano ("Impact of Nanoscale Topography on Genomics and Proteomics of Adherent Bacteria"), we showed for the first time that a pure topographical cue, namely a highly uniform gold nanostructured substrate, may play per se a significant role in determining important biological responses (in terms of morphological, genetic, and proteomic profile changes) in the model organism Escherichia coli. | |
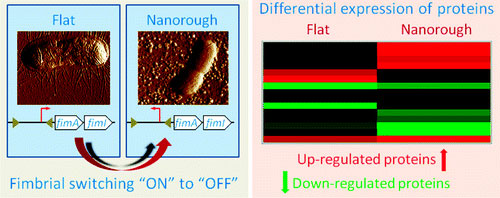
| In particular, we found by Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM) that E. coli adhering onto nanostructured gold substrates undergo an important morphological change, namely the loss of fimbriae. Fimbriae are arm-like adhesive organelles which enable bacteria to adhere onto the host's tissue cells, leading to a pathogenic event, but they are also fundamental for the attachment onto abiotic surfaces and for the starting of biofilm development. On the other hand, E. coli growing onto classical glass slides or flat gold films (used as control experiments) normally express fimbriae. | |
 |
|
| Highly controlled nanostructured substrates impact the bacterial behavior in terms of morphological, genomic, and proteomic response. (Reprinted with permission from American Chemical Society) | |
| Such intriguing finding was also confirmed by real-time quantitative PCR (RT-qPCR) analyses of bacteria growing onto flat and nano rough surfaces. We demonstrated, in fact, that the nanostructured gold substrates lead to a genetic fimbrial switching of the fimbrial operone: while bacteria growing onto flat surfaces have the fimbrial operon in the "ON" orientation (thus allowing the transcription of all fimbrial subunits), E. coli adhering onto nano rough gold surfaces have the fimbrial operon in the "OFF" orientation (thus the transcription of the fimbrial subunits is prevented). | |
| Furthermore, we investigated the entire proteomic profile of bacteria growing onto flat and nano rough substrates, by 2D Differential in Gel Electrophoresis (2D-DIGE) and Mass Spectrometry (MS) analyses, revealing that a significant number of protein involved in protein biosynthesis, protein transport, metabolic pathway, and DNA repair system were differently expressed. | |
| In particular, our results showed that bacteria adhering onto nano rough surfaces undergo general stress processes, with a consequent overexpression of enzymes involved in the protection of DNA, amino acid synthesis, energy production, regulation, and rearrangement of the external membrane. At the same time, E. coli down-regulates some transport proteins and enzymes related to DNA synthesis, possibly trying to avoid mistakes and/or damages during base synthesis while adhering onto nano rough surfaces. | |
| Our findings mainly highlighted two important issues: First, a nanoscale variation in surface topography may cause important biochemical responses in E. coli, such as the loss of fimbriae and modulation of its protein expression pattern. Second, in addition to the physicochemical investigations, that were typically carried out in previous works to explain the bacteria-surface interaction event, deep biochemical and molecular biology approaches are absolutely required to study the interaction processes of bacteria with inorganic surfaces. | |
| We believe that the understanding of the bacterial responses and adaptations to nanostructured surfaces will open interesting perspectives for the design of novel and more efficient implant devices with unanimously certified antibacterial characteristics. | |
| For this reason, our next efforts will be addressed to a deeper understanding of the relationships between nanostructured surfaces and the genetic pathways involved in the cell-to-cell communications (i.e., quorum sensing molecules), and biofilm formation. | |
| Provided by Center for Bio-Molecular Nanotechnology, Italian Institute of Technology (IIT), as a Nanowerk exclusive | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
