| Posted: May 24, 2013 | |
A pH-responsive nanoscale vaccine with tailored immune responses |
|
| (Nanowerk Spotlight) Reporting in the journal ACS Nano ("pH-Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides"), researchers have developed nanoscale polymer micelles that elicit both humoral and cellular immunity. The constructs could help in the fight against infectious diseases and cancer. | |
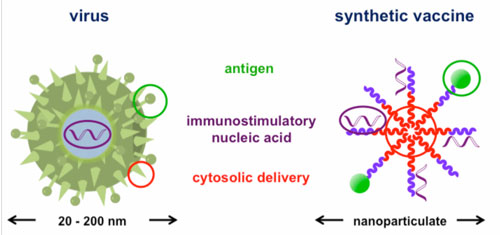
| Nanomaterials hold promise as synthetic vaccines. They have the ability to deliver cargo to specific immune cells and modulate the resulting immune response. Compared to natural vaccine vectors, including engineered viruses or attenuated pathogens, synthetic nanoscale vaccines are safer, more controlled, and have the potential to be more effective. Nanoscale vaccines may also prevent, or even treat a wider range of diseases, including cancer. Yet, while many nanoscale vaccines promote humoral immunity, few can prime the immune system to search for and destroy infected cells directly. | |
| Humoral immunity refers to the production of antibodies by specialized immune cells. Antibodies mark foreign or pathogenic material so it can be more easily recognized by the immune system. While antibodies are a key component of the immune response, they can only act on targets that are outside of cells. However, many pathogens avoid immune recognition by residing within cells. To fight these infections, the immune system generates specialized cells, known as ‘cytotoxic T-cells’, which recognize and eliminate infected cells. | |
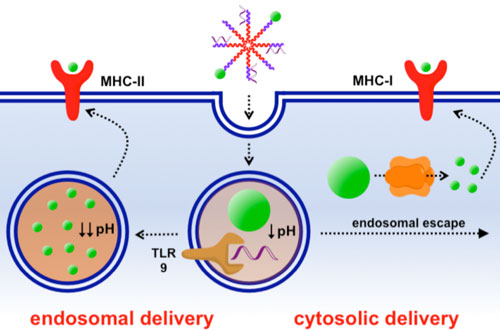
| Most nanoscale vaccines deliver antigens – the molecules that the immune system recognizes - to ‘antigen presenting cells’ (APCs). Once a nanomaterial vaccine is internalized within APCs, it is usually sequestered within small vesicles called endosomes’ If the nanomaterial remains within the endosome, the antigen is typically processed in such a way that the immune system generates antibodies against it. To generate cytotoxic T cells, the nanomaterial must escape from the endosome and deliver the antigen into the cell cytoplasm. | |
 |
|
| The synthetic copolymer micelles used in this study mimic the structure of natural viruses. | |
| As part of their natural infection cycle, certain pathogens, including the influenza virus, can escape from endosomes and enter the cell cytoplasm. Often, these pathogens use pH-dependent molecular switches that destabilize the endosome membrane in response to the acidic environment within the endosome. Inspired by nature’s approach to escaping the endosome, Dr. Patrick Stayton and his colleagues at the University of Washington incorporated a pH-responsive polymer into nanoscale polymer micelle vaccines to facilitate antigen delivery to the cell cytoplasm. | |
| The polymer micelles were engineered out of block co-polymer chains, which are formed by ‘fusing’ together two polymer chains with distinct compositions. One portion of the polymer chain was formed from a hydrophobic, pH-responsive block composed of a combination of ionic and hydrophobic groups, while the other was composed of hydrophilic cationic chemical groups. When the polymer chains were placed in water, the hydrophobic portions associated with one another, forming a flower-like nanostructure that is about 30nm in diameter. | |
| The surface of the micelles, known as the ‘corona’, is composed of the hydrophilic portions of the polymer chains. To turn the micelles into vaccines, the researchers loaded them with a model antigen, ovalbumin. Dr. John Wilson, a post-doctoral fellow at the University of Washington and first author on the study explains the choice. “Ovalbumin doesn’t have many direct clinical applications, but it is a common standard for the initial design and testing of vaccines. It was used here as a model so we could test how well the vaccine works at a proof-of-concept level.” Ovalbumin was incorporated into the micelles through engineered disulfide linkages in the polymer corona. Inside the cells, the disulfide bonds are broken and ovalbumin is released so that the cell can process it. | |
| The authors also loaded the micelles with an adjuvant. The immune system does not mount a defense against the adjuvant directly. Instead, the adjuvant helps to ‘prime’ the immune system to respond to the antigen. “We used bacterial DNA motifs, known as ‘CpG sequences’ as an adjuvant,” explains Wilson. “Because they are composed of a phosphate backbone, the negatively charged CpG sequences were easily incorporated into the corona of the micelles by spontaneous electrostatic interactions.” | |
 |
|
| Nanoparticle vaccines based on pH-responsive polymers deliver antigen to the cytoplasm to enhance cellular immunity. They can also deliver contents to the endosome, which is important for generating humoral immune responses. By delivering antigen by both the cytosolic and endosomal pathways, these vaccines allow immune responses to be tuned. | |
| The researchers tested the efficacy of the micelle vaccines by injecting them under the skin of mice. Several weeks after inoculation, they found that the micelles elicited both cytotoxic T-cells and a robust antibody response against ovalbumin. Incorporating both the antigen and adjuvant into the micelles was critical. A physical mixture of the two generated a far weaker immune response. | |
| Generating simultaneous humoral and cellular immunity is important, since it primes the immune system to fight disease using complementary strategies. “It also shows that micelle design can be used to tune the immune response,” adds Wilson. | |
| The polymer micelle design is well suited for eventual clinical translation. The polymer chains are synthesized using ‘RAFT’ polymerization, which is reproducible and easy to scale-up. Once the polymer chains are synthesized, they can be self-assembled into micelles spontaneously in water. This avoids the more complex manufacturing processes associated with liposomes or polymeric nanoparticle vaccines. In addition, in their assembled form, the micelles are less than 30nm in diameter, so they can be sterile-filtered. | |
| Because the individual polymer chains are small, it is likely that they will be excreted fully from the body. This is a significant advantage over nanoscale vaccines made from inorganic nanostructures (like gold nanoparticles) that remain in the body for an extended period of time and may lead to long-term toxicity. | |
| Although vaccines based on the polymer micelles developed by Stayton’s group won’t reach the clinic in the immediate future, this study is an important first step towards that goal. Moving forward, Stayton and his colleagues intend to test their vaccines against more clinically relevant diseases. The researchers are also looking at the effect of changing the micelle properties to further control the immune response. “We’ve found interesting results so far using micelles with neutral or anionic corona chemistries,” explains Wilson. Ultimately, the ability to tailor the immune response to nanomaterial vaccines holds promise for preventing and treating diseases including hepatitis, HIV, and even cancer. | |
| John Wilson will soon be joining the Department of Chemical and Biomolecular Engineering at Vanderbilt University to start his own independent research program. He is actively recruiting post-doctoral fellows and graduate students. Interested candidates are encouraged to contact him directly ([email protected]). | |
|
By Carl Walkey, Integrated Nanotechnology & Biomedical Sciences Laboratory, University of Toronto, Canada.
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
