| Posted: Jul 26, 2013 | |
Highly effective tumor targeting platform with nanoghosts |
|
| (Nanowerk Spotlight) The field of drug discovery is growing at a remarkable pace, leading to the development of many new drugs, most of which are generally more potent than their predecessors, yet suffer from poor solubility and/or high toxicities. Targeted drug-delivery vehicles (e.g., liposomes, nano-particles) have often been proposed in an effort to reduce the side effects of such drugs and improve their overall efficacy for treating genetic, viral and malignant diseases. | |
| Three main considerations must be addressed when designing any such delivery system: It should be biocompatible; bioavailable; and highly selective to its specific target. | |
| Targeting may be improved by conjugating drug carrying vehicles with targeting moieties that substantially improve their selectivity. For example, antibodies, proteins etc. have been incorporated into nano-sized drug-carriers made from polymeric particles, micelles or liposomes, yet their relatively short circulation time and the complexity of their production render them too costly and inefficient. | |
| The need for drug delivery vehicles is particularly stressed in cancer treatment, where high doses of toxic drugs are often required. Passively-targeted drug-loaded vehicles are still the predominantly used delivery systems for cancer therapy. Because of their nano-size and physical properties, such systems were shown to achieve extended circulation times, and retention in the tumor microenvironment—owing to the Enhanced Permeability and Retention (EPR) effect of tumor vasculature and microenvironment. These systems, nonetheless, are limited by tumor vascularization and permeability that are largely dependent on the stage of the malignancy and tumor type. Consequently, active targeting vehicles, once a promising therapeutic approach, have almost exhausted their potential, particularly in the area of cancer therapy where such solutions are desperately needed. | |
| In our recent paper ("Reconstructed Stem Cell Nanoghosts: A Natural Tumor Targeting Platform") we report on a novel targeted drug-delivery vehicle for cancer therapy, which can selectively target the tumor niche while delivering an array of therapeutic agents. | |
| This targeting platform is based on unique vesicles ('nanoghosts') that are produced, for the first time, from intact cell membranes of stem cells with inherent homing abilities, and which may be loaded with different therapeutics. | |
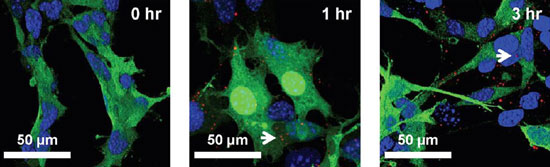 |
|
| Binding of nanoghosts (white arrow) to PC3 cells; cell, green (GFP); nucleolus, blue (DAPI) evaluated using confocal microscopy over short (3 h) incubation times. (Reprinted with permission from American Chemical Society) | |
| We have shown that such vesicles, encompassing the cell surface molecules and preserving the targeting mechanism of the cells from which they were made, can outperform conventional delivery systems based on liposomes or nanoparticles. | |
| These vesicles leverage the benefits related to the size, and chemical and physical properties of nano-liposomes, allowing them to efficiently entrap various hydrophilic and hydrophobic drugs, and be administered through different routes while exhibiting versatile and controllable release profiles. The prior art pertaining to the design of this unique and novel drug-delivery platform is drawn from and associated with the production and utilization of cell-derived vesicles, and the inherent tumor-targeting abilities of mesenchymal stem cells (MSCs). | |
| A similar therapeutic effect, to what we have achieved, was previously demonstrated for prostate cancer, using monoclonal antibodies against N-cadherin, which is highly expressed in castration-resistant prostate cancer; however, it requires more frequent and higher dosing. | |
| Our therapeutic outcome is comparable to that demonstrated by De Marra et al. ("New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study") who used no less than three administrations per week of liposomes encapsulating Zoledronic acid and exceeded the effect achieved by a weekly administration of an imatinib–mitoxantrone liposomal formulation. | |
| The efficiency of our delivery system is even more compelling in light of the results reported by Srivastava et al. ("Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo"), which demonstrated no inhibition of tumor growth after two weeks and as many as four IV administrations of similar quantities of free sTRAIL. The efficacy of our system also exceeded that of previously reported liposomal formulations containing sTRAIL tested on glioblastoma and lung cancer. | |
| Till now, nanoghosts made from mammalian cells have been used to study cell membranes and were utilized for cancer immunotherapy but have never been tested as targeted drug-delivery vehicles. Recently, we reported a novel concept describing a targeted drug-delivery system based on nanoghosts, which were prepared from the outer cell membranes of a non-human cells engineered to express the human receptor (CCR5) of a viral ligand (gp120) found on the surface of HIV-infected cells ("Cell derived liposomes expressing CCR5 as a new targeted drug-delivery system for HIV infected cells"). Drug-loaded nanoghosts selectively targeted HIV-infected cells in vitro, achieving over 60% reduction in their viability compared to empty nanoghosts, free drug, or nanoghosts applied on control uninfected cells that were not affected at all. | |
| This intrinsically-targeted system, which does not entail the elaborate production of targeting molecules and their incorporation into passive vehicles, represents a simpler and more clinically relevant approach than existing particulate drug-delivery vehicles. | |
| Our success in using nanoghosts to target HIV-infected cells has prompted us to devise a more sophisticated universal and non-immunogenic delivery platform, in which the nanoghosts will be produced from stem cells that are known to naturally target various tumors. | |
| Insights gained from this work may pave the way for new research utilizing nanoghosts’ inherent targeting to treat not only tumors but also sites of inflammations, wound healing, and trauma. | |
| The knowledge accumulated on the entrapment of diverse drugs can facilitate the loading of nanoghosts with nucleic acid (DNA, siRNA etc.) for gene therapy. | |
| Nanoghosts loaded with MRI contrast agents (Indocyanine or magnetite nano-crystals) can open unique research avenues in imaging and diagnosis. Their small size and specific targeting abilities may enable the nanoghosts to freely travel in the body possibly detecting small and sub-metastatic cancer nuclei and lesions, which are otherwise undetectable using conventional methodologies. | |
| Owing to MSCs natural role in regenerative medicine, nanoghosts can be also investigated in tissue engineering applications for delivering growth factors for regenerating tissues. | |
| Finally, MSCs can be engineered to express additional targeting molecules and used to treat other diseases manifested by the expression of unique targetable ligands. | |
| This work was conducted by PhD students Naama Ester Toledano-Furman, Yael Lupu-Haber, Limor Kaneti, and the Lab manager Dr. Tomer Bronshtein. | |
|
By Marcelle Machluf, Associate Professor, The Faculty of Biotechnology & Food Engineering, Technion - Israel Institute of Technology, Haifa, Israel.
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
