| Posted: Oct 09, 2013 | |
Covetics - Rare new hybrid fuses nanocarbons and metal in bond stronger than sp2 |
|
| (Nanowerk Spotlight) Thomas Edison likely created the first commercial carbon fiber when he produced high-resistance carbonized filaments by heating cotton sewing thread and bamboo slivers to power an incandescent light bulb. His lamp shed light for 600 hours introducing the first reliable, marketable light bulb and laying the foundation for a new field in chemistry that would be introduced more than a century later with the invention of covetics. This new class of materials also marks a game-changer for engineers and designers that have long sought to combine high-strength carbon with metal in their pursuit to improve metal’s performance. For the first time the hybrid fuses nanocarbons and metal in a bond that is stronger than graphene-like sp2 carbon bonds. | |
| To create covetics, co-inventors Jason Shugart and Roger Scherer, Ph.D., developed a new method of carbon catalyzation which uses molten metal and metal alloys as an ionizing medium. Nanocarbon structures form in situ while bonding to the metal ionizing medium. | |
| Initial testing revealed that covetics responded to physical deformation more like polymers than metals. In addition Shugart and Scherer found they could control the chemical reaction and the ingredients to tailor covetics to end-use applications. | |
 |
|
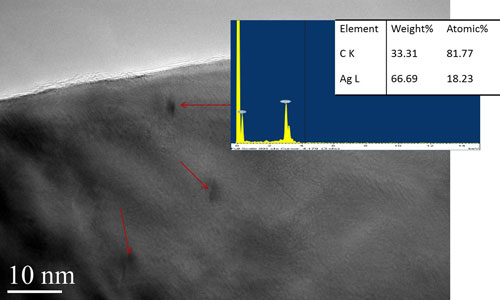
| A high magnification image of Ag cv 3% with energy dispersive (EDS) spectrum. The dark regions marked by arrows are domains with high C content. Some of these domains are faceted. (Image: Dr. Lourdes Salamanca-Riba, University of Maryland) | |
| Much like graphene, called “the next wunderkind material” because of its extraordinary properties, the potential uses and advantages associated with covetics are seemingly endless. “We knew immediately that we had something,” says Shugart. Shugart is president of Waverly, Ohio-based Third Millennium Materials LLC (TM2). | |
| Nanocarbons and graphene remain on the cutting edge of material science and are continuing to pick up speed. “In recent years carbon nanotube manufacturers have produced composites with metal and nanocarbons – including graphene, carbon nanotubes, aggregated diamond nanorods, buckyballs and nanodiamonds,” says Shugart. “The problem with every composite that has been produced is that these materials cannot survive remelt for easy fabrication or recycling. Covetics are the first bonded nanocarbon-metal materials with significantly enhanced properties that can survive repeated melting cycles.” | |
| To date, the hybrid has demonstrated the ability to withstand more than 1500 degrees Celsius under an oxygen plasma lance without separation of carbon and metals. Shugart adds that because covetics are carbon compounds that use different metals, potential users will have to gain an understanding of carbon and its effect on the properties and behavior of the material. | |
| Covetics promise to deliver superior properties for advanced engineering applications but the hybrid presently defies classification and the nature of its chemical bond is still a mystery. The material continues to confound universities, national laboratories and the military. Despite years of traditional tests and analysis, these organizations could neither qualify nor quantify the presence of carbon in covetics. It wasn’t until 2007 that a Chicago-based materials testing laboratory was able to confirm the presence of carbon using Transmission Electron Microscopy. “So the question then became: what form of carbon is it?” says Shugart. | |
| Dr. Lourdes Salamanca-Riba, professor of materials science and engineering at the University of Maryland, found the answer in 2011. She first heard about covetics in 2010 from Dr. David Forrest at the Naval Surface Warfare Center, Washington Navy Yard, DC. “He asked me if I’d like to take a look at something brand new,” she says. “I expected to see particles of carbon in the host metal (copper) but I could see no evidence of it. That led me to conclude that the carbon was not readily visible because it had somehow bonded into the lattice structure of the metal. That’s when covetics became much more interesting.” | |
 |
|
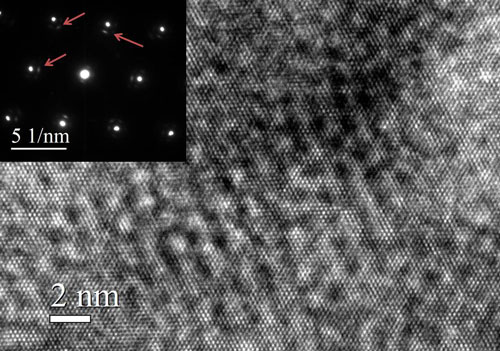
| A high resolution lattice image (HRTEM) from Cu cv 2% with its electron diffraction pattern. The image shows the atomic planes of copper (fine scale spacing) and a modulation superimposed on the copper planes. The modulation is a result of the C incorporation. And it is along preferred crystallographic directions of copper. (Image: Dr. Lourdes Salamanca-Riba, University of Maryland) | |
| Dr. Salamanca-Riba’s investigation included among other things use of an Energy Dispersive X-ray and Electron Energy Loss Spectroscopies. The spectroscopy employed high energy electrons which induce electronic excitations within the sample’s atoms producing a signature energy that identified the element producing it. | |
| “These techniques gave us our first glimpse of the presence of carbon in the material even though we could not see particles,” she says. Dr. Salamanca-Riba spent much of the first two years of her research working with copper covetic and some aluminum and silver covetic. In January 2011 she chose to focus solely on silver covetic. “Silver has fewer contaminants than copper,” she says. “We detected graphene-like carbon, amorphous carbon and other carbon structures in the silver covetic.” | |
| “The properties of covetics are dominated by the properties of the carbon structures created during the chemical reaction,” says Harry Couch, chief technology officer for TM2. “Silver covetic responds to physical and mechanical loadings that are superior to either polymers or metals.” | |
| TM2 was awarded U.S. patent 8,349,759 on January 8, 2013 for the metal-carbon composition and the invention of silver covetic. Five additional patents will be issued in 2013 for copper, gold, zinc, tin and lead covetics. To date, TM2 owns the intellectual property for covetics based upon 15 elements. | |
| Dr. Salamanca-Riba’s ongoing work with covetics is focusing on understanding the role of nanocarbons on the structure and properties of metals, how the bonding process between carbon and metal occurs and discover the mechanism that makes the bond so strong. Testing and application work with the military have already demonstrated the hybrid’s ability to improve thermal and electrical conductivity and yield strength and resist corrosion and oxidation. | |
| “Covetics represents a new material,” says Dr. Salamanca-Riba. “The structure of the material changes significantly. It also behaves quite differently. One of the most amazing things is that Third Millennium Materials has incorporated high concentrations of carbon in metals in a way that was not possible before. It’s a new type of bond between carbon and the metal host.” | |
|
By Louis Luedtke, CEO for Third Millennium Metals LLC ([email protected])
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
