| Posted: Jan 21, 2008 | |
New carbon nanotube hydrogen storage results surpass Freedom Car requirements |
|
| (Nanowerk Spotlight) Two of the major challenges of our modern, mobile society are the shrinking of available fossil energy resources on one hand and climate change associated with global warming on the other. Continuing population growth multiplied by the increase in consumption and living standards, especially in developing countries, will require more and more oil, coal and natural gas to 'power' humanity. Notwithstanding efforts like the Kyoto Protocol - which wasn't signed by the two major CO2 polluters China and the U.S. - an ever increasing rate of fossil fuel usage means that the increasing emission of CO2 is likely to cause an acceleration of the climate change that is in progress already. Transportation, in particular passenger cars, is one of the areas where new technology could lead to environmental beneficial change. Never mind that GM is still selling 15-20,000 Hummers a year, or that Tata is planning to sell millions of its new Nano car. One of the much touted technological solutions is to substitute fossil hydrocarbon based energy with the energy from carbon-free sources like the sun, nuclear energy, or the hot interior of the Earth and use hydrogen as an energy carrier (read more about this in "The Hydrogen Economy"). Hydrogen can be produced from water using energy from carbon-free sources [editor's note: unfortunately it can also be produced from dirty fossil fuels; see the Nanowerk Spotlight "Nanotechnology could clean up the hydrogen car's dirty little secret"] and can serve as fuel in fuel cells to generate electricity, either stationary or on board of vehicles. Considerable research efforts are going into the evaluation of various nanostructures, such as carbon nanotubes, to find the most suitable hydrogen storage materials. | |
| Safe, efficient and compact hydrogen storage is a major challenge in order to realize hydrogen powered transport. According to the DOE Freedom CAR program roadmap the on-board hydrogen storage system should provide 6 weight % (wt%) of hydrogen capacity to be considered for the technological implementation. | |
| Currently, the storage of hydrogen in the absorbed form is considered as the most appropriate way to solve this problem. Thus, a media capable of absorbing and releasing large quantities of hydrogen easily and reliably is being actively sought. Since claims by Dillon et al. that single-walled carbon nanotubes (SWCNT) can store hydrogen, this material has been considered as a candidate for hydrogen storage media ("Storage of hydrogen in single-walled carbon nanotubes"). | |
| Physisorption and chemisorption both have been proposed as possible mechanisms for hydrogen storage in carbon nanotubes. While most of the previous studies have focused on the hydrogen storage through physisorption, recent Density Functional Theory (DFT) calculations for single-walled carbon nanotubes (SWCNT) indicate the potential for up to 7.5 wt% hydrogen storage capacity for this material through chemisorption by saturating the C-C double bonds in the nanotube walls and forming C-H bonds (see "Generalized Chemical Reactivity of Curved Surfaces: Carbon Nanotubes" and "Theoretical evaluation of hydrogen storage capacity in pure carbon nanostructures"). However, direct experimental evidence of the high values of the hydrogen capacity through chemisorption has not been demonstrated yet. | |
| In this regard, we studied the chemical interaction of hydrogen with carbon nanotubes using such techniques like X-ray Photoelectron Spectroscopy (XPS) and X-ray Absorption Spectroscopy (XAS). These methods allow us to observe the formation of C-H bonds through the modification of the local electronic structure around specific carbon atoms and to quantify the amount of hydrogen that is chemically adsorbed in terms of per carbon atom. | |
| For our experiments we used two different types of as grown SWCNT thin films (T1 and T2) with different diameter distributions. The T1 SWCNT film had an average diameter around 1.6 nm and the average diameter for the T2 SWCNT film sample was equal to 2.0 nm. We also used in situ atomic H-beam treatment of the studied samples for the hydrogenation to escape complexities related to the H2 molecule dissociation. It was demonstrated before that atomic hydrogen treatment of SWCNT film does cause the formation of C-H bonds ("Hydrogenation of Single-Walled Carbon Nanotubes"). | |
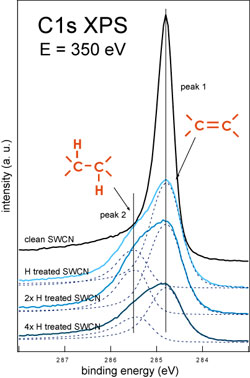
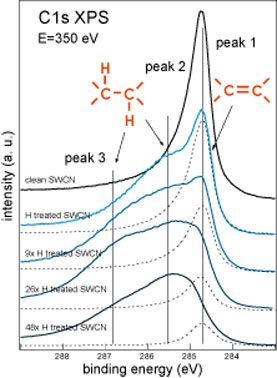
| Using C1s XPS as a probing tool we studied the interaction of the atomic hydrogen with T1 and T2 samples of SWCNT. The C1s XPS spectra measured during hydrogenation sequence for T1 and T2 samples are shown in figure 1 below. | |
  |
|
| Figure 1. C1s XPS spectra measured during the hydrogenation sequence of T1 SWCNT (left) and T2 SWCNT (right) samples. (Credit: Anton Nikitin, SSRL) | |
| These measurements demonstrate dramatic differences in the behavior of the different nanotube samples under the H treatment. In the case of the T1 sample H treatment allows to hydrogenate ∼30% of the carbon atoms in the walls of the nanotubes. Additional doses of the atomic hydrogen do not increase the degree of the sample hydrogenation but cause the etching of the material that is directly indicated by the C1s line shape and intensity dependences on the total H dose. | |
| In the case of the T2 sample the H treatment can hydrogenate the surface of the SWCNT film up to much higher values before hydrogenated SWCNT become unstable and can be etched under the beam of atomic hydrogen. | |
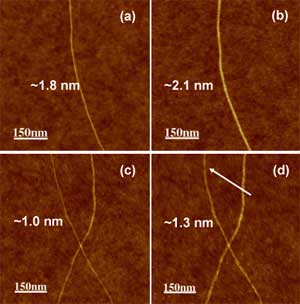
| The observed difference in the maximal degree of the hydrogenation between T1 and T2 samples are due to the differences in the diameter distribution of the nanotubes. It was observed before that SWCNT with smaller diameter are less resistant to the hydrogen plasma induced etching ("Hydrogenation and Hydrocarbonation and Etching of Single-Walled Carbon Nanotubes"). Using single nanotube AFM measurements (see fig. 2 below) we also found that the same H treatment dose causes the appearance of the cuts in the thinner nanotubes while the thicker ones preserve integrity. | |
 |
|
| Figure 2. AFM images of the same tubes before and after hydrogenation. (a) Before hydrogenation, a SWCNT with the diameter of ∼1.8 nm; (b) diameter of the tube in (a) increased to ∼2.1 nm after hydrogenation; (c) before hydrogenation, a SWNT with diameter of ∼1.0 nm; (d) the tube in (c) is cut after hydrogenation (marked with arrow) and diameter of the tube increased to ∼1.3 nm. (Credit: Anton Nikitin, SSRL) | |
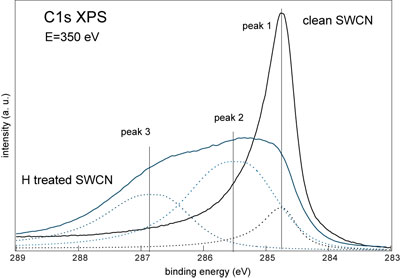
| The decomposition of the C1s line measured from the highly hydrogenated T2 sample (see fig. 3 below) provided the information about the exact degree of the maximal hydrogenation value for this material. The ratio between peak 1 (clean carbon atoms) and peak 2 and 3 (hydrogenated carbon atoms) is 1 to 10. If we assume that there is one hydrogen atom per one hydrogenated carbon atom then the observed more than 90% hydrogenation corresponds to more than 7 wt% of hydrogen storage capacity. This value is already above the requirement in the Freedom CAR Hydrogen Storage Technologies Roadmap goal for 2010. | |
 |
|
| Figure 3. The decomposition of XPS C1s spectrum of the H treated T2 sample at maximal hydrogenation degree. For the comparison C1s spectrum of clean T2 SWCNT sample is shown. (Credit: Anton Nikitin, SSRL) | |
| Using XPS as a probing tool we also studied the hydrogen desorption from the hydrogenated SWCNT. Our results showed that most of the C-H bonds dissociate in the temperature range between 200°C and 300°C. The DFT based modeling of the H recombinative desorption mechanism demonstrated that the H desorption temperature is mainly controlled by the reaction kinetics and nanotube curvature can be used to tune the energy of C-H bonds in the hydrogenated SWCNT to minimize the energy overhead to form hydrogenated SWCNT. | |
| The present results indicate that for certain types of SWCNT the hydrogen chemisorption can provide more than 7 wt% of hydrogen storage capacity and the optimal C-H bond energetics can be tuned by the choice of the nanotube curvature range to minimize the energy losses of the hydrogen desorption/adsorption process. | |
| To fully realize hydrogen storage in SWCNTs it is essential to find means to hydrogenate SWCNTs using molecular hydrogen. A possible way to do this is to use the so-called spillover process. In this case, the H2 molecules dissociate at the surface of catalyst nanoparticles deposited on the nanotube surface and H radicals spill over from the catalyst to the surface of the nanotubes and form C-H bonds. It has been shown that the presence of platinum nanoparticles inside nanotube materials results in a 5-fold increase of the hydrogen uptake (Lee, Y.-W.; Clemens, B.M. Appl. Phys. J., submitted). | |
| Thus we can assume that by choosing non-bundled nanotubes with appropriate diameter distribution, optimizing the nanostructure of deposited catalyst and by choosing appropriate hydrogenation temperature to enhance H species diffusion along nanotube surface we can use the spillover process to form the H-SWCNT complexes without compromising the hydrogen weight capacity of the material. | |
| Our work ("Hydrogen Storage in Carbon Nanotubes through the Formation of Stable C-H Bonds") was supported by the Global Climate Energy Project and carried out at the Stanford Synchrotron Radiation Laboratory, national user facility supported by the U.S. Department of Energy, Office of Basic Energy Sciences. | |
| By Anton Nikitin, Surface Science and X-Ray Spectroscopy Group, Stanford Synchotron Radiation Laboratory (SSRL). Copyright Nanowerk LLC | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
