| Posted: Sep 04, 2018 | |
Ion generation and ion trapping in liquid crystals by means of nanomaterials |
|
| (Nanowerk Spotlight) Nowadays, practically everyone is familiar with liquid crystal devices (just think of liquid crystal displays (LCD) in your favorite laptop, smartphone or TV). The reorientation of elongated liquid crystalline molecules under the action of the applied electric field is a major physical effect enabling the use of liquid crystals in a variety of applications. | |
| By reorienting liquid crystalline molecules, basic physical properties of liquid crystals (for example, colors of liquid crystal pixels making up an LCD) can be tuned in a controllable way. | |
| To improve liquid crystal devices, new liquid crystal-forming materials are required. Recently, by merging liquid crystals and nanotechnology, a new, non-synthetic way to produce advanced liquid crystal materials was proposed. In short, it was achieved by dispersing various types of nanomaterials in liquid crystals. | |
| In this case, the designed composite material (liquid crystals + nanodopants) can exhibit the combined functionality of liquid crystals and nanodopants, often unachievable by means of chemical synthesis. For example, among other things, we can imagine such exotic materials as ferroelectric and ferromagnetic nematic liquid crystals. | |
| As mentioned above, typically, liquid crystal devices are driven by electric fields. Therefore, it is very important to understand how nanomaterials in liquid crystals can affect their electrical properties, including the behavior of ions in such systems. | |
| Ions in liquid crystals are always present in minor quantities. They are very undesirable objects because of the so-called screening effect. In the case of display applications, this effect can result in image sticking, image flickering, high driving voltage, and overall slow response of the device. | |
| Given the utmost importance of this problem, the effects of nanomaterials on the behavior of ions in liquid crystals were studied by numerous independent research groups (interested readers can refer to a recent review [1] analyzing more than 100 original research papers). | |
| An entire database of existing experimental data suggests that, in general, nanomaterials in liquid crystals can behave as either ion-capturing objects (in this case, nanodopants capture mobile ions in liquid crystals thus reducing their concentration) or as ion-releasing objects (in this regime, nanomaterials increase the concentration of mobile ions in liquid crystals by releasing some ions from their surface). | |
| In some cases, no effect on the concentration of mobile ions in liquid crystals can also be observed. All these experimentally observed regimes and the possibility of switching between them were poorly understood until now. Recently published papers [2,3] reports the model of nanomaterials in liquid crystals as ion-generating and ion-capturing objects and apply the proposed model to existing experimental results. | |
| Major physical factors that are enabling three different regimes in liquid crystals doped with nanomaterials – ion-trapping, ion generation, and no change regimes – are either intrinsic or extrinsic ionic contamination of nanomaterials, quantified by means of the dimensionless contamination factor, νNP. | |
| Very often, this important factor is overlooked by researchers, thus leading to seeming contradiction between the reported results. | |
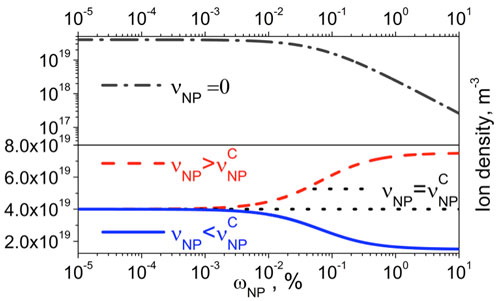
| It should be noted, that in the case of zero contamination factor (νNP = 0; it means 100% pure nanoparticles) the only possible outcome is the ion capturing regime shown in Figure 1 (dashed-dotted curve): the concentration of mobile ions in liquid crystals doped with nanomaterials decreases as their concentration goes up. | |
| On the opposite side, 'contaminated' nanoparticles can result in three different regimes shown in Figure 1: ion-capturing (or ion trapping) regime (solid curve), ion-releasing (or ion generation) regime (dashed curve), and no change regime (dotted curve) [2]. | |
 |
|
| Figure 1: Liquid crystals doped with nanoparticles: the ion density as a function of the weight concentration of nanodopants, ωNP The case of 100% pure (νNP = 0) nanoparticles in liquid crystals is represented by dashed-dotted curve (ion trapping regime). Different regimes of contaminated nanoparticles in liquid crystals are also shown: ion generation (or ion releasing) regime (dashed curve); ion trapping (or ion capturing) regime (solid curve); no change regime (dotted curve) [2]. (Reproduced under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work [2] is properly cited) | |
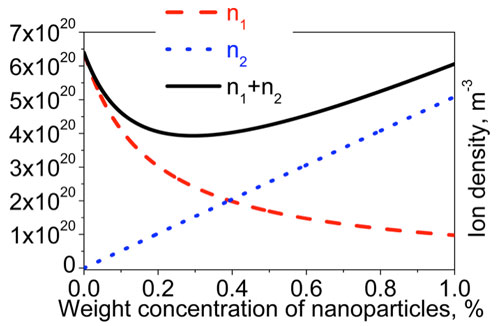
| The proposed model of ion-generating and ion-capturing nanomaterials was discussed further in paper [3]. Among other interesting features, by considering two dominant types of ions in liquid crystals doped with nanoparticles, experimentally observed non-monotonous behavior can also be modelled as shown in Figure 2. | |
 |
|
| Figure 2: The total ion density of mobile ions in liquid crystals doped with nanoparticles as a function of their weight concentration ωNP: n(ωNP)=n1(ωNP)+n2(ωNP) (solid curve); n1(ωNP) (dashed curve) and n2(ωNP)(dotted curve) [3]. (Reproduced under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work [3] is properly cited) | |
| Findings reported in [2,3] have important practical and scientific implications. First of all, nanomaterials in liquid crystals should be treated as ion-generating or ion-capturing objects. | |
| Recently, this idea was generalized to account for the presence of ion-generating and ion-capturing substrates in liquid crystal devices [4]. From academic perspectives, it implies the need for dedicated studies of the nature of the contamination factor of nanomaterials. | |
| As was pointed out in [2,3], in general, this ionic contamination can be either intrinsic or extrinsic. Extrinsic ionic contamination can be reduced by revisiting synthetic and handling procedures used during the production, storage, and handling of nanomaterials. | |
| Intrinsic contamination of nanomaterials is caused by their chemical nature, for example, self-dissociating nanoparticles generating ions as a result of their chemical dissociation. | |
| To make better use of liquid crystals and nanotechnology, we should broaden our understanding of physical mechanisms involved in the generation and capturing of ions in liquid crystals doped with nanomaterials. | |
References |
|
| [1] Y. Garbovskiy, I. Glushchenko, Nano-Objects and Ions in Liquid Crystals: Ion Trapping Effect and Related Phenomena, Crystals, 5(4), 501-533 (2015). doi:10.3390/cryst5040501 | |
| [2] Yuriy Garbovskiy, Nanoparticle-Enabled Ion Trapping and Ion Generation in Liquid Crystals, Advances in Condensed Matter Physics, Volume 2018, Article ID 8914891, 8 pages https://doi.org/10.1155/2018/8914891 | |
| [3] Yuriy Garbovskiy, Nanomaterials in Liquid Crystals as Ion-Generating and Ion-Capturing Objects, Crystals 2018, 8(7), 264; https://doi.org/10.3390/cryst8070264 | |
| [4] Yuriy Garbovskiy, Time-dependent electrical properties of liquid crystal cells: unravelling the origin of ion generation. Liquid Crystals 2018, 45, 1540. https://doi.org/10.1080/02678292.2018.1455228 | |
|
By Yuriy Garbovskiy, PhD, a researcher at the UCCS BioFrontiers Center & Department of Physics, University of Colorado
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
