| Jan 21, 2019 | |
Super-stable antinomy carbon composite anodes to boost potassium storage performance |
|
| (Nanowerk Spotlight) Potassium-ion batteries (PIBs) have been considered as promising alternatives to lithium-ion batteries due to the rich natural abundance of potassium (K) and similar redox potential with Li+/Li. | |
| However, due to the large K ion radius and slow reaction dynamics, the previously reported PIB anode materials (carbon-based materials, alloy-based anodes such as tin and antimony, metal oxides, etc.) suffer from a low capacity and fast capacity decay. | |
| In order to achieve a high capacity and excellent cycle stability for K storage process, rational design of the electrode materials and proper selection of the electrolytes should be considered simultaneously. | |
| Recently, two research teams led by Prof. Chunsheng Wang and Prof. Michael R. Zachariah from the University of Maryland, College Park, have designed and fabricated a novel antimony (Sb) carbon composite PIB anode via a facile and scalable electrospray-assisted strategy and found that this anode delivered super high specific capacities as well as cycling stability in a highly concentrated electrolyte (4M KTFSI/EC+DEC). | |
| This work has been published in Energy and Environmental Science ("Super Stable Antimony-carbon composite anodes for potassium-ion batteries"). | |
 |
|
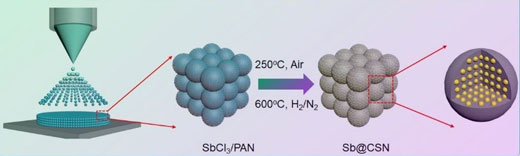
| Figure 1. Schematic illustration of electrospray-assisted strategy for fabricating antimony @carbon sphere network electrode materials. (© Royal Society of Chemistry) | |
| We have successfully fabricated a novel antimony carbon composite with small Sb nanoparticles uniformly confined in the carbon sphere network (Sb@CSN) via a facile and scalable electrospray-assisted strategy. | |
| Such a unique nanostructure can effectively mitigate the deleteriously mechanical damage from large volume changes and provide a highly conductive framework for fast electron transport during alloy/de-alloy cycling process. | |
| Alongside the novel structural design of the anode material, formation of a robust solid-electrolyte-interphase (SEI) on the anode is crucially important to achieve its long-term cycling stability. | |
| The formation of a robust SEI on the anode material is determined by both the surface chemistries of active electrode materials as well as electrolyte compositions such as salt anion types and concentrations. | |
| Therefore, designing a proper electrolyte is extremely important for the anode to achieve a high cycling stability. | |
| In our study, we have for the first time developed a stable and safe electrolyte of highly concentrated 4M KTFSI/EC+DEC for PIBs to promote the formation of a stable and robust KF-rich SEI layer on an Sb@CSN anode, which guarantees stable electrochemical alloy/de-alloy reaction dynamics during long-time cycling process. | |
 |
|
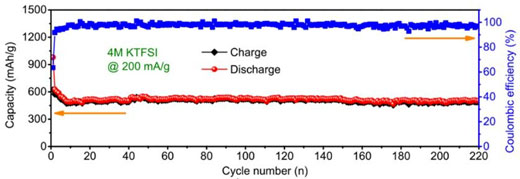
| Figure 2. Cycling performance of antimony carbon sphere network electrode materials at 200mA/g current density in the highly concentrated electrolyte (4M KTFSI/EC+DEC). (© Royal Society of Chemistry) | |
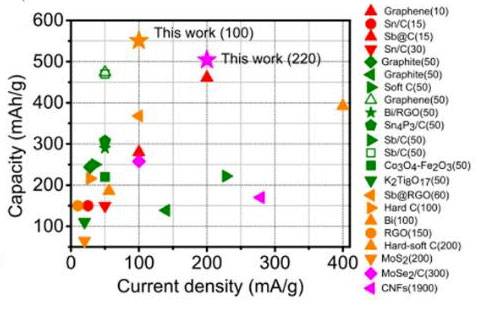
| In the optimized 4M KTFSI/EC+DEC electrolyte, the Sb@CSN composite delivers excellent reversible capacity of 551 mAh/g at 100 mA/g over 100 cycles with a capacity decay of 0.06% per cycle from the 10st to 100th cycling and 504 mAh/g even at 200 mA/g after 220 cycling. This demonstrates the best electrochemical performances with the highest capacity and longest cycle life when compared with all K-ion batteries anodes reported to date. | |
| The electrochemical reaction mechanism was further revealed by density functional theory (DTF) calculation to support such excellent Potassium-storage properties. | |
 |
|
| Figure 3. Capacity comparison of Sb@CSN anode with previous reported anodes in potassium ion batteries. (© Royal Society of Chemistry) | |
| In conclusion, these outstanding performances should be attributed to the novel nanostructure of Sb nanoparticles uniformly encapsulated into conductive carbon network and the formation of a more stable and robust KF-rich SEI layer on Sb@CSN in the optimized 4M KTFSI electrolyte. | |
| These encouraging results will significantly promote the deep understanding of the fundamental electrochemistry in Potassium-ion batteries as well as rational development of efficient electrolyte systems for next generation high-performance Potassium-ion batteries. | |
|
Yong Yang, Research Associate, Prof. Zachariah Research Group, Department of Chemical and Environmental Engineering, University of California, Riverside
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
