| Sep 21, 2020 | |
4D nanomedicine based on biodegradable and biocompatible nanoalloys: difficult as catching a comet! |
|
| (Nanowerk Spotlight) Imagine catching a comet. | |
| Comets are clusters of frozen gases passing close to the Earth at high speed, whose fate is to gradually evaporate until disappearing. Hence, it is not easy to capture one (the hunter should be very fast…) and it is not possible to observe a comet for very long. | |
| Now imagine the comet is as small as a few billionths of meters and it is made of elements from the periodic table that do not like each other; whose fate is that of separate in space and dissolve in biological environments or just in air. Before this happens, the elements are trapped in what is called a non-equilibrium alloy nanoparticle. | |
| Since this nanoalloy changes over time, it is not enough to provide its composition and size at a given time, but one should know also how it will change in the near future, or how it changed in the near past. | |
| A way to describe this behavior is to say that it is a 4D nanosystem, where the traditional three-dimensional shape evolves along a 4th dimension that is time. | |
| Why is it important to catch this '4D nano-comet'? Because, contrary to real comets, metastable nanoalloys promises to improve current capabilities in the diagnosis and treatment of cancer and other diseases. | |
| Nanomaterials can revolutionize current medical procedures but only a tiny fraction of the thousands of nanomedicines proposed to date are progressing towards clinical applications. | |
| The limiting factor is in the 'nanomedicine dilemma': only those nanoparticles with sizes of tens of nanometers are efficiently accumulated in target sites without the need for a large excess of drug dose; whereas ultrasmall nanoparticles below that size are cleared from the body in a short time, which minimizes the interaction with tissues and immune system. | |
| Ideally, nanomedicines should behave as a 4D material, capable of size reduction only after use to allow rapid body clearance. | |
| As for comets, the hunter of 'nano-comets' must be very fast. In a recent article published in ACS Nano ("4D Multimodal Nanomedicines Made of Nonequilibrium Au-Fe Alloy Nanoparticles"), the nanoalloys were achieved by a quick process running in non-equilibrium conditions, called 'laser ablation in liquid' (LAL). | |
| LAL proved to be an efficient and versatile synthesis technique for freezing elements in non-equilibrium structures, produced free of chemical contaminants and with an environmentally friendly procedure. LAL also has the advantage of a self-running set-up that can be controlled remotely, for instance with a smartphone or a tablet. | |
 |
|
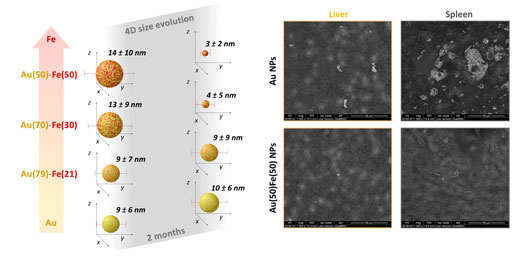
| Left: Sketch of the 4D behavior measured for Au-Fe alloys with increasing iron content after two months in physiological environment. By increasing the Fe content above a certain threshold, the 4D behavior is observed as a size reduction due to nanoparticles self-degradation. Right: Representative environmental scanning electron microscopy images of liver and spleen slices collected two months after administration of either pure Au (top) or Au-Fe alloy (bottom) nanoparticles to murine models. The animals treated with Au nanoparticles still show large agglomerates of gold, that are absent in the animals treated with Au-Fe nanoalloys, due to the self-degradation and facilitated clearance of the 4D nanomedicines. (Image: Vincenzo Amendola) (click on image to enlarge) | |
| This study evidenced the main parameters relevant to catch non-equilibrium nanoalloys with appealing features for a 4D nanomedicine, such as being composed of biocompatible Au and Fe elements only. | |
| Then, the 4D behavior of the nanoalloys was monitored over time in conditions of relevance for biomedical applications, and the best class of nanoparticles for the realization of 4D nanomedicines was identified in Au-Fe alloys with equimolar composition. | |
| Successively, the authors verified the ability of the selected nanoalloys to provide advantages for widespread bioimaging techniques like magnetic resonance imaging (MRI) and x-ray computed tomography (CT) in animal models. | |
| Importantly, the nanoalloys were monitored over a period of two months in animals, and their biodistribution compared to that of benchmark equivalents like pure Au nanoparticles or iron-oxide contrast agents approved for clinical use. | |
| The results showed that the 4D Au-Fe nanomedicines were able to leave the organism in a shorter time compared to 'standard' nanoparticles, without side effects. | |
| These results open the way to the easy realization of inorganic contrast agents, drug delivery carriers, or sensitizers for radiotherapy that are safe and have limited biopersistence, as required for clinical use. | |
|
Provided by Università di Padova as a Nanowerk exclusive
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
