| Nov 13, 2020 | |
Core/shell nanoparticles as efficient reducing agents |
|
| (Nanowerk Spotlight) Semiconductor nanoparticles, also known as quantum dots (QDs), have been studied for their size-tunable electronic and optical properties for over 30 years. Applications have been demonstrated for biological sensing and imaging, as well as for electronic devices such as LEDs and low-cost solar cells. | |
| Recent endeavors have examined the efficacy of QDs as redox agents. This allows them to reduce H+ to generate solar fuels from sunlight and to effect other organic transformations such as CO2 reduction (Read more: ACS Energy Letters, "Designing the Surfaces of Semiconductor Quantum Dots for Colloidal Photocatalysis"). | |
| Unfortunately, core QDs are unstable because they tend to precipitate in solution in a matter of hours which results in surface damage that renders the nanoparticles useless. Concerning the use of QDs as fluorescent sensors or for LEDs, this well-known problem can be solved by overcoating the core with an inorganic shell. | |
| Such core/shell dots are more robust due to their literal insulation from the environment. However, this insulation minimizes the redox activity of the semiconductor. | |
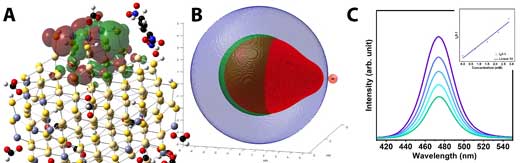 |
|
| Atomistic (A) and course-grained (B) models of CdZnS and CdZnS/ZnS, respectively, reveal QD hole states that are closely paired to a surface-bound reduced organic substrate. The result is a high Stern-Volmer quenching constant (C) for the core/shell QD compared to the core material alone. (Image courtesy of the researchers) (click on image to enlarge) | |
| As recent report from a team of researchers from the University of Illinois at Chicago (UIC) and US Army researchers from Ft. Belvoir has turned this paradigm upside down, as they demonstrate core/shell nanoparticles can be engineered to display enhanced redox activity towards organic substrates. | |
| As published in the journal Nanoscale ("Charge carrier pairing can impart efficient reduction efficiency to core/shell quantum dots: applications for chemical sensing"), CdZnS quantum dots were being developed as fluorescent sensors for high energy (i.e. explosive) chemicals such as RDX and TNT. | |
| To their surprise, the material was found to be more effective towards TNT reduction when passivated by a ZnS shell, by a factor of 300%. This observation prompted further experimental and theoretical investigations. | |
| They found that the inorganic passivation prevents trapping of the semiconductor’s electron and hole by surface defects, allowing the quantum dot to remain in the excited state for a longer period of time. | |
| This in turn provided more opportunity for a substrate to diffusively interact with the semiconductor, upon which it is reduced. | |
| One mystery remained – while the enhanced excited state lifetime was shown to enhance substrate reduction by the passivated QD, the inorganic shell should have nonetheless put a stop to redox activity. | |
| This problem was studied theoretically; using the supercomputer facilities at UIC the group found that the near-degeneracy of the valence states in core CdZnS and the ZnS shell allows the hole to easily cross over from the core into the shell. | |
| This means that the electron on the surface-bound substrate and the hole in the shell can remain in close contact, which minimizes the energy of the state. | |
| The researchers posit that this close interaction must stabilize the reduced substrate, which counters the effect of the shell’s electronic barrier. | |
| The unexpected demonstration of enhanced redox activity of core/shell CdZnS/ZnS quantum dots will impact on several scientific fronts. Many new analytical platforms are being developed that rely on QD reduction and oxidation as signal transduction mechanisms. | |
| Furthermore, QDs are being sought after as suppliers of electron and hole charge carriers for biological and chemical processes; many of these are relevant to alternative energy and chemical syntheses. | |
| These activities may benefit from the use of CdZnS/ZnS QDs, or from a design that allows for charge carriers to remain in close contact upon reduction or oxidation of a preferred organic substrate. | |
| Provided by the University of Illinois at Chicago as a Nanowerk exclusive | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
