| Mar 12, 2021 | |
Delivering semiconductor quantum dots into live cells |
|
| (Nanowerk Spotlight) Researchers at the University of Illinois at Chicago (UIC) achieved a milestone in exploring biology using nanotechnology as recently reported in the journal Nanoscale ("Cytosolic delivery of membrane-penetrating QDs into T cell lymphocytes: implications in immunotherapy and drug delivery"). | |
| A consortium of research groups led by Prof. Ying Hu of the UIC Chemistry department, with Profs. Preston Snee and Anne George also of UIC, utilized single-particle tracking to investigate the interaction between human T cells and individual fluorescent nanoparticles of semiconductor quantum dots (QDs). | |
| The researchers were able to deliver QDs into the cytosol of live T cells by decorating the nanoparticles with a unique cell-penetrating peptide. | |
 |
|
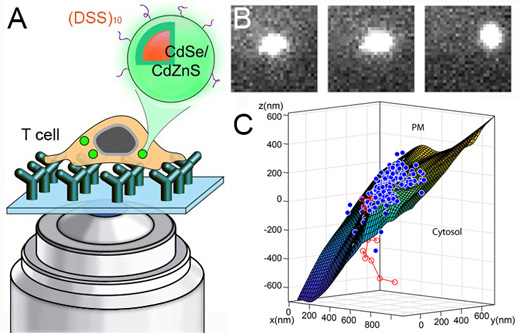
| Figure 1. (A) CdSe/CdZnS quantum dots (QDs) coated with a cell penetrating peptide can be delivered to the cytosol of live T cells. (B) Single particle images of the internalized QDs allow researchers to track them in three dimensions as they cross the plasma membrane as shown in (C). (Image courtesy of the researchers) | |
| Quantum dots have long held the promise of enhancing biological discovery from imaging studies due to their highly robust optical properties. They are 10× more absorptive than organic dyes and are nearly impervious to photobleaching. However, their adoption by the biological imaging community has been derailed by the fact that the nanoparticles are very difficult to deliver into the cytosol of live cells. | |
| Application of the many protocols that exist to deliver cargo such as DNA have been found to be, at best, highly inefficient when applied to QDs. | |
| An initial breakthrough came from the lab of Prof. Anne George. Her group has spent years studying dentin phosphophoryn, which is a highly phosphorylated protein found in the extracellular matrix of dentin. They found that the protein is a very efficient cell penetrating peptide, and as such in 2013 the George and Snee groups collaborated to demonstrate the delivery of the QDs to the nucleus of MC3T3-E1 cells as reported in the Journal of Biological Chemistry ("Acidic Domain in Dentin Phosphophoryn Facilitates Cellular Uptake"). This was accomplished by attaching a chimera of dentin phosphophoryn and a nuclear localizing factor to the surface of quantum dots. | |
| While highly successful, the issue remained that the technology was difficult to transfer to other research groups. | |
| When Prof. Hu joined UIC in 2018, his group initiated the effort of finding a smaller biological delivery vehicle for quantum dots that could be prepared using a peptide synthesizer. | |
| To this end, the George group’s previous characterizations of dentin phosphophoryn revealed that it was composed of repeat units of aspartic acid followed by two serines, i.e. (DSS)x. This allowed them to synthesize a cell penetrating peptide, (DSS)10, that was attached to water-soluble quantum dots. | |
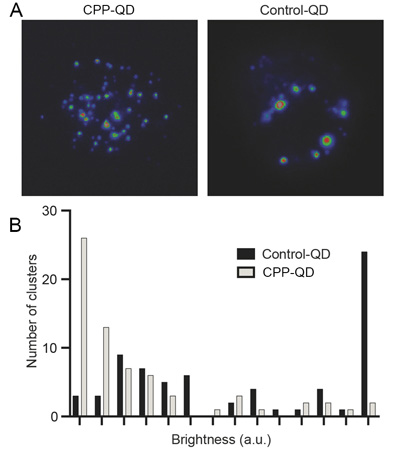
| These imaging agents were applied to live T cells (white blood cells that are part of the immune system) and studied at the single quantum dot level by the Hu Group. The results were a smashing success; they demonstrated cytoplasmic delivery of the QDs by their free diffusion and lack of aggregation compared to control samples. | |
| They also found that the functionalized nanoparticles entered T cells through an alternative pathway distinct from endocytosis, thereby allowing the nanoparticles to access the cytoplasmic machinery. | |
 |
|
| Figure 2. (A) Fluorescent images of CPP-functionalized QDs and controls after internalization into live T cells. (B) A histogram of the intensities of CPP-QDs and controls inside live cells reveals that the functionalized quantum dots are freely diffusing while the control dots are aggregated. (Image courtesy of the researchers) | |
| The study paves the way for improving drug delivery and immunotherapy using novel nanocarriers. | |
| Furthermore, targeted delivery of genetic materials into T cell lymphocytes holds the promise to boost T-cell-mediated immunity which can help protect against COVID and cancer. | |
| Provided by University of Illinois at Chicago as a Nanowerk exclusive | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
