| Jan 26, 2022 | |
Technological improvements in lubricant development due to recent advances in nano additives |
|
| (Nanowerk Spotlight) Lubricants are essential for optimal equipment efficiency and performance. However, in order for the latter to be achieved, lubricants have to be well developed and optimized. Hence, many tribological research studies are being conducted to advance lubricant technology. | |
| This article will discuss the use of nanotechnology in lubricants. Nanoparticle additives show significant enhancements in lubricant attributes like anti-oxidation capability, tribological features, and thermal properties. Nanotechnology offers the possibility of using nanosized additives to increase the performance of lubricating oil. The addition of nanoparticles to conventional base oils is a promising method for improving properties like friction and wear resistance in instruments. | |
Introduction |
|
| Tribology is a science dedicated to lubrication, and encompasses how lubricants affect the friction and wear between contacting surfaces. Tribological research primarily aims to understand the mechanisms by which lubrication occurs. | |
| Comprehension of such lubrication mechanisms is vital as it enables researchers the ability to predict friction and wear trends, to gauge the effect of altering lubrication conditions, or to compare various lubricant formulations. | |
| Tribological research is integral towards promoting energy efficiency, longevity, and durability for the machinery of countless industries including automotive, paper, and steel. Recent studies indicate that advances in tribology could lead to savings of around 11% of total energy loss in power generation, transportation, and industrial processes [1]. | |
| Optimal lubricant or grease formulations work by maintaining smooth contact between surfaces, thus reducing the overall energy loss due to friction. However, available lubricants can oftentimes be inapplicable or inefficient for certain applications, for example, applications at extreme pressures, temperatures, or contact loads. Improper lubrication, corrosion/oxidation, or thermal runoff can subsequently result in lubricant degradation and thus machinery failure [2]. | |
| Furthermore, the advancements in base oil technology are reaching an upper limit in terms of tribological and thermal properties. Additives, therefore, have greatly risen in importance as a supplement to widen the range of applicability while simultaneously improving lubrication and anti-wear properties in a lubricant. Although dispersants and detergents were the additives of choice for quite some time, nanoparticles, such as WS2 and MoS2, have demonstrated outstanding effects as solid lubricant additives to traditional base oil lubricants [3][4]. | |
| Among the advantages of using nano-additives are their suitable sizes to enter contact asperities, thermal stability, variety of particle chemistries, and the ability to react with the surface without an induction period, an important feature for conventional lubricant additives [2]. | |
| Contrarily, proper dispersion and suspension stability in base oil lubricants remain a prominent challenge in the incorporation of nano additives. A comprehensive understanding of how various nano additives affect lubricant properties and the underlying mechanisms which vary with morphology, concentration, and size is required in order to adequately benefit from their effectiveness. | |
Key Mechanisms for Lubrication with nanoparticle additives |
|
| Many studies on nanoparticle additives are still in debate in terms of explaining the mechanistic effect through which nano additives improve lubrication performance. Numerous research groups have proposed a number of lubrication mechanisms consistent throughout multiple tribological studies through surface analysis techniques, including scanning electron microscopy/energy dispersive spectrometry (SEM/EDS), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS) [5]. | |
| Ball-Bearing Effect | |
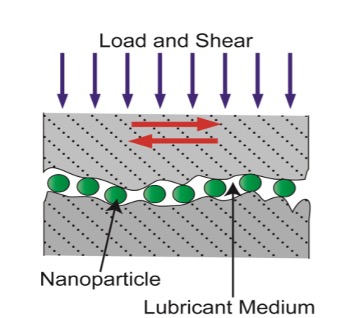
| The ball-bearing effect is most typically observed in spherical or quasi-spherical nanoparticles [5]. If the size of the nanoparticles exceeds the surface roughness, they can roll between the surfaces when sheared against one other, as depicted in Figure 1. They may become stuck between the asperities and lose their lubricity if this happens. | |
| As a result, the friction mechanism shifts from sliding to a combination of rolling and sliding, reducing friction and wear. Low-load conditions must be maintained between the rubbing surfaces in order for the nanoparticles' shape and stiffness to be kept, which is one of the prerequisites for this mechanism to work. | |
 |
|
| Figure 1. Illustration of the ball-bearing effect. (Kheireddin 2013) | |
| Tribo-film Formation | |
| There is also the protective film formation, also referred to as tribo-film [5]. The tribological behaviors of interacting surfaces are governed by tribo-films and near-surface materials. The reaction between the treated material and the additives in the given environment triggers the creation of this film [6]. | |
| The development of such films is a complicated phenomenon that has sparked a lot of research into their mechanism and composition. Rapoport et al. found that dispersing IF-WS2 nanoparticles in lubricating oil improved tribological properties by forming a physical protective coating composed of distorted nanoparticles depositing on the surfaces. [5]. | |
| Liu et al. also reported the creation of a tribochemical coating on rubbed surfaces as a result of the deposition of chemical reaction products created during shearing action [5]. | |
 |
|
| Figure 2. Schematic diagram of the lubrication mechanism of silica nanoparticle dispersed in PAO. (Sui et al. 2015) | |
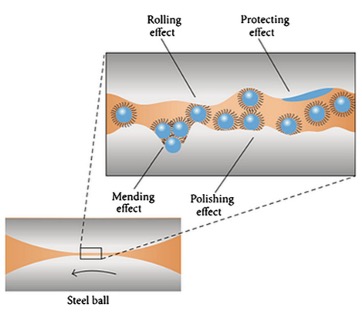
| Polishing and Mending Effect | |
| Another method is the polishing effect or the smoothing out process. It has been hypothesized and experimentally proven that nanoparticles can fill in the valleys between the asperities in a process known as smoothing out [5]. Because of the reduced surface roughness, this "artificial smoothing" technique will improve tribological qualities [5]. | |
| A reduction in surface roughness would contribute to less friction and thus less wear. Additionally, nanoparticles can also impart a mending or self-repairing effect, which is characterized by the deposition of nanoparticles on interacting surfaces compensating for mass loss [7]. The nanoparticles deposit on the worn surface minimize abrasion during this process. | |
| Nanoparticles suspended in lubricating oil have the ability to fill friction surface scars and grooves. Figures 1 and 2 depicts the antiwear and friction-reduction mechanisms of nanoparticles as lubricant additives. When lubricants carried nanoparticles into the contacting area, they filled in microgrooves on the metal surface (mending effect) and polished rough metal surfaces (polishing effect) [8]. Both of these processes have the potential to reduce surface roughness, hence lowering friction and wear. | |
Effects of Size and Structure |
|
| Nanoparticles size in lubricants have three different types of effects. To begin with, the size of nanoparticles determines their intrinsic mechanical and physicochemical properties, which directly relate to their tribological properties [1]. | |
| When nanoparticles with a tougher surface than the shearing surfaces are placed between the latter, the surfaces indent and scratch. As a result, while designing nanoparticle-based lubrication systems, the size-induced differences in the hardness of nanoparticles must be considered. | |
| When nanoparticles squeeze out of the contact region during loading and shearing, the shearing surfaces do not benefit from the tribological properties of nanoparticles [1]. To protect shearing surfaces, nanoparticle-based lubrication solutions must remain in the contact zone throughout loading and shearing. | |
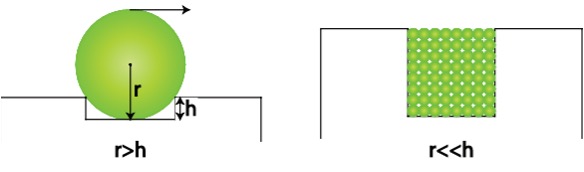
| As the asperities of shearing surfaces can operate as physical barriers that retain nanoparticles inside the contact zones, the effect of nanoparticle size can come into play [1]. Two separate conceptual scenarios can be considered to demonstrate this impact. | |
 |
|
| Figure 3. The effect of surface roughness on the organization of nanoparticles between the asperities of shearing surfaces. (Akbulut 2012) | |
| The ratio of root mean square (RMS) roughness to nanoparticle radius is a measure of how much lateral force is necessary to dislodge a nanoparticle out of an asperity barrier in the first scenario, when the characteristic roughness length scale of shearing surfaces is smaller than the radius of the nanoparticles as shown in Figure 3 [1]. | |
| If the nanoparticles are excessively big and non-adherent, they can readily exit the contact zone, resulting in inadequate lubrication. The valleys between asperities of the shearing surfaces can be filled with nanoparticles in the second case, when the characteristic roughness length scales (h) are much larger than the radius of the nanoparticles; nanoparticles artificially smooth out the shearing surfaces and, thus, can improve the tribological properties (Figure 4) [1]. | |
 |
|
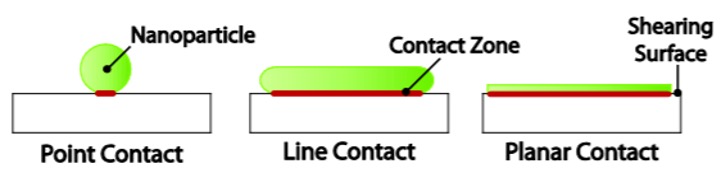
| Figure 4. The effect of nanoparticle shape on the contact pressures upon loading. (Akbulut 2012) | |
| Finally, lubricant homogeneity is strongly influenced by colloidal stability when it comes to the ability of the lubricant to function reliably and smoothly [1]. It is important to know the rate at which nanoparticles settle out of dispersion because this is an important parameter that determines colloidal stability. | |
| Nanoparticle shapes are another important factor to consider when designing lubricant additives based on nanoparticles [1]. There are several shapes (structures) of the nanoparticles, including platelets, spherical solids, spherical multilayered particles and nanoparticles with hollow core. Spherical solid particles scratch or indent the surface, but if particles have hollow core they deform and absorb the shock [9]. Usually multilayered structures do not scratch/polish the surface, as interlayer forces are weak, and they exfoliate under shear or shock forces, thus reducing friction. | |
| In addition, as nanoparticles are loaded, their shape directly determines the pressures they experience. As illustrated in Figure 4, a nanosphere would experience the most pressure for a given load, while a nanosheet would experience the least pressure. The reasoning behind this is that nanospheres make point contact with a counter surface, while nanoplatelets make a planar contact [1]. Therefore, nanosheets are the least likely to indent and deform asperities of shearing surfaces for a given load. | |
| Another important distinction to establish is the difference between solid and hollow core nanospheres. Incorporating a hollow core in fact prevent indentation as they are much more flexible and allow for the absorption of load [10]. | |
Manufacturing of Nanoparticle Additives |
|
| Solid-phase Techniques | |
| Technology has been developing in the manufacturing of nanoparticle additives that can be used in lubricants. One prominent technique is nanoimprint lithography (NIL) [11]. To nanoimprint a surface, three basic components are necessary: a stamp with appropriate feature sizes, the material to be printed, and printing equipment with an adequate temperature of 90 °C to 100 °C, pressure in the range of about 50 to 100 bar, and control of parallelism of the stamp and substrate. | |
| NIL is a biocompatible, flexible, and low-cost fabrication technology. As a result, NIL has an advantage over traditional nanofabrication techniques, and various versions have been developed. NIL is available in numerous forms, including the most common parallel technique, a sequential process known as Step-and-Stamp Imprint Lithography (SSIL), and roll-to-roll NIL [11]. | |
| Step and flash imprinting lithography (SFIL) is a technique in which no pressure is applied and instead a UV (ultraviolet) polymer curing process is introduced into the sequence. NIL is a method for fabricating organic optoelectronics and sensors. By patterning 400 nm gratings to a depth of 20 nm on a conjugated polymer, this approach is utilized to produce organic light-emitting diodes. Data storage, bioelectronics, and nanofluids are examples of applications. | |
| The ultimate resolution of NIL is determined by the stamp's minimum feature size. Electron beam lithography and dry etching are commonly used for high-resolution stamps, whereas metal lift-off is used for shallow stamps. Si or SiO2, which is occasionally electroplated, is the preferred material [11]. Stamps on wafer-size scale have been studied by many groups depending on the use. The design of the stamp is intrinsically linked to the polymer to be printed. | |
| Polymers used for this process include Poly (Methyl Methacrylate) (PMMA), Polystyrene (PS) polymers, mr-I-8000, mrI-9000, and mr-L6000 [11]. To achieve uniform thickness of the residual layer and patterned features over the sample area, it is critical to keep the stamp parallel to the substrate throughout the procedure. This process is illustrated in Figure 5. | |
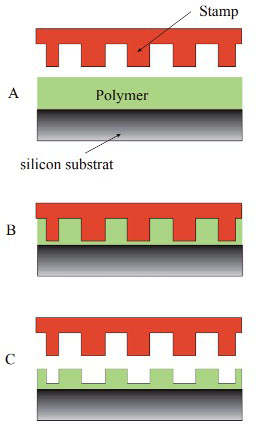 |
|
| Figure 5. Schematic of the principles of Nanoimprint lithography. The heated sample and stamp (A), stamp and sample are brought into contact and pressure is applied (B), the system is cooled down and stamp is separated from the sample. (Nowosielski et al. 2006) | |
| Another solid-phase technique is mechanical attrition, a method for creating new alloys and phase mixtures from powder particles that have been developed as an industrial process since 1970. This approach can overcome the quantity limits of nanocrystalline preparation, allowing for the large-scale production of nanocrystalline powders. | |
| Mechanical milling has the advantage of being able to operate at low temperatures, allowing freshly produced grains to expand slowly. It is feasible to create advanced materials with specific grain or interface-boundary designs using this technology. Researchers working with mechanical attrition are focusing on nanocrystalline nanomaterials with grain or interphase barriers between nanophase domains. | |
| Gaseous-phase Techniques | |
| Furthermore, gaseous methods are also prominent methods for industrial nanoparticle manufacturing, particularly using a system of solid or liquid particles suspended in air or another gaseous environment known as aerosol [12]. Aerosol-based nanoparticles can be made by either atomizing a solution with a specific composition to generate tiny droplets that crystallize into solid nano-constructs when the solvent is evaporated or by aero-to-particle conversion via condensation and coagulation, respectively [13]. | |
| Nebulization or electrohydrodynamic atomization can be used to atomize many types of solutions, while spark discharge, flame, furnace, or plug-in-play plasma processes can be used to prepare aero-to-particles [13]. Particles can range in size from a few molecules to 100 micrometers. | |
| Another gas phase technique is atomic or molecular condensation (gas condensation). In this technique, a stream of vaporized and atomized materials is sent to a chamber filled with either an inert or reactive gas environment after a bulk material, mainly metal, is heated to a sufficiently high temperature (far beyond the melting point but less than the boiling point) within a vacuum chamber [12]. | |
| The gas pressure is strong enough to stimulate particle formation, yet low enough to allow spherical particle formation. The nucleation and subsequent production of nanoparticles are caused by the rapid cooling of metal atoms caused by collisions with gas molecules. | |
| Additionally, laser ablation technology utilizes a high-power laser beam with an optical focusing system and a metal-target feeding device [12]. The laser beam is focused on the target's surface, and a plume of evaporated material is ejected perpendicular to the target's surface, extending into the gas region above the target. The carrier gas transports the produced particles to the product collector. | |
| Liquid-phase Techniques | |
| There are also liquid phase techniques [12]. A chemical approach based on hydrolysis or condensation processes is the sol-gel process. The sol???gel process entails two steps: (1) hydrolysis of the precursor in acidic or basic mediums, and (2) polycondensation of the hydrolyzed products. After this, the sol-gel process works using chelating agents. The chelating agent, usually a polymer, reacts with metal ions. Metal ions are trapped by the chelating agent in the condensation nets of the gel structure [14]. While this occurs, the microstructure of the polymer is strongly impacted by the pH value. | |
| Nanosized particles precipitate when the number of reactants used correspond to the appropriate mole ratio [15]. Low temperatures during processing, adaptability, and ease of shape and embedding are only a few of the benefits of sol-gel methods. | |
| Finally, there is sonochemistry, a field of study in which molecules undergo chemical reactions as a result of the use of high-intensity ultrasound radiation. Acoustic cavitation, which involves the development, growth, and collapse of bubbles in an ultrasonically irradiated liquid, is the driving force behind the sonochemical process. | |
| It should be noted that carbon-based materials are also common in the production of nanomaterials [12]. Methods of synthesis for fullerenes include the gas combustion method, in which a continuous low flow of hydrocarbon fuel is burned at low pressure in the presence of oxygen, and the arc discharge of graphite electrodes in the presence of helium at 200 Torr, resulting in yields of up to 70% of C60 fullerenes and 15% of C70 fullerenes. The first method has the benefit of producing huge clusters of fullerenes with a size distribution that can be easily controlled by adjusting combustion conditions. | |
Challenges |
|
| As nanotechnology continues to develop, it still has quite a few challenges. Some of them include dispersion stability issues, purity, defects, and environmental concerns. One of the most difficult tasks tribological researchers are faced with is ensuring uniform dispersion throughout the grease base oil [16]. | |
| A useful lubricant requires a steady suspension of nanoparticles. The ability of nanoparticles to lubricate the contact region is hampered by their aggregation. Lubricant???s tribological performance deteriorates as the concentration of nanoparticles is increased. This is due to weakening interfacial interaction between the additives and the lubricants. The weak interfacial interaction causes the full potential of using nanoparticles as lubricant additives to be severely constrained. | |
| Two significant variables to improve dispersion stability are to solve the compatibility problem between nanoparticles and non-aqueous solvents, as well as to reduce the adhesion and agglomeration problem between particles. | |
| Adsorption of a surfactant, particularly polymers, onto the surface of nanoparticles can improve their compatibility with non-aqueous liquids. Adsorption of a surface modifier on a particle's surface works as a bridge between nanoparticles and the base fluid, increasing the steric resistance between particles and allowing nanoparticles to be dispersed in non-aqueous solvents [17]. | |
| Many researchers have successfully dispersed nanoparticles in non-aqueous solvents and kept the dispersion stable for a long time using this method. Also, nanoparticles in a dispersed environment begin to condense when the van der Waals attraction between them is higher than other repulsive forces [17]. It is hard to break down huge clusters or aggregates back into primary particles after they have formed. Physical methods include ultrasonication, mechanical stirring, high-shear mixing, ball milling, and high-pressure homogenization [18]. | |
| Chemical techniques refer to the use of chemical agents to chemically change nanoparticle surfaces in order to improve dispersion stability [18]. | |
| Surface modification agents, such as organic modification agents and silane coupling agents, primarily address the issue of inorganic nanoparticle stabilization in base oils. Organic compounds utilized as modifying agents typically have polar groups and long alkyl chains that can chemically adsorb onto inorganic nanoparticles, allowing for uniform dispersion of inorganic nanoparticles in an organic solvent. Physical suspension methods are environmentally friendly, easy, and cost-effective, and have been widely employed in prior studies, however, nanoparticles treated physically are prone to secondary agglomeration and are unstable in lubricants [18]. | |
| Although chemical approaches can considerably improve dispersion stability, chemical agents impair the natural characteristics of nano additives while also affecting the tribological properties of nano lubricants [18]. As a result, self-dispersed methods for preparing a stable and uniform suspension must be developed. | |
| Another challenge is the purity of nano additives. Many nanotube forms leave a lot of metallic impulse behind, which can be hazardous to work with [19]. One method of purification of nanoparticles is diafiltration [20]. Diafiltration has a lot of promise for the effective and convenient purification and size separation of water-soluble nanoparticles since it allows for the removal of small-molecule contaminants as well as the separation of tiny nanoparticles from larger nanostructures all in one step, using ultrafiltration membranes. | |
| Although nano additives may be playing an indispensable role in enhancing mechanical efficiency, they may have detrimental effects on the environment. For instance, Zinc dialkyldithiophosphate is the most common anti-wear and friction reducer (ZDDP) [21]. Despite its widespread use, ZDDP has been observed to degrade catalysts in catalytic converters, and the sulfur emissions from ZDDP constitute an environmental concern. | |
| The prospect of taking environmental protection into account motivates the development of more efficient and environmentally friendly lubricant additives, such as polymer brush-grafted nanoparticles and oil-soluble hairy nanoparticles [21]. | |
Conclusion |
|
| To operate machinery and equipment, all processes and industries require a considerable quantity of energy in the form of electricity or fuel, which is amplified by the presence of friction. Machine efficiency is improved by reducing the amount of energy lost due to friction. | |
| Friction-induced wear is also a key cause of failure of critical engineering components in systems such as pin-joints, engines, power generators, wind turbine gears, aerospace parts, bearings, camshafts, pumps, and so on. Tribological inadequacies cause energy and material losses in practically every mechanical device in use, and they account for a considerable amount of the cost of any country's economy. Oil sources are declining and energy prices are continuing to rise [22]. | |
| Advancements in lubricants would result in gains in mechanical efficiency leading to large energy and cost savings. Economic investigations have led experts to believe that proper tribology R&D funding in countries like the US, UK, China, and Canada, may result in a 1.0 to 1.4% increase in gross national product [22]. | |
| While the importance of nanotechnology in lubricants has been highlighted, it must be further researched not only to improve mechanical efficiency and economies but also due to address health factors. Apart from all the benefits nano additives bring, they may also have unintended negative consequences on human health and the environment, as the quantum mechanics that govern nanomaterials interactions with other substances also make toxicological behavior difficult to predict. | |
| Further research must be conducted to understand to a greater extent the prevalent processes and effects of nano additives on industrial uses. As the industry continues to develop, the nanotechnology aspect of it must be further advanced in order to avoid holding back the utilization of utmost instruments. | |
References |
|
| [1] Akbulut, Mustafa. "Nanoparticle-based lubrication systems." J. Powder Metall. Min 1.1 (2012): 1-3. | |
| [2] Shahnazar, Sheida, Samira Bagheri, and Sharifah Bee Abd Hamid. "Enhancing lubricant properties by nanoparticle additives." International journal of hydrogen energy 41.4 (2016): 3153-3170. | |
| [3] An, Vladimir & Irtegov, Yuri & De izarra, Charles. (2014). Study of Tribological Properties of Nanolamellar WS2 and MoS2 as Additives to Lubricants. Journal of Nanomaterials. 2014. | |
| [4] Kalin, Mitjan, Janez Kogov??ek, and Maja Rem??kar. "Mechanisms and improvements in the friction and wear behavior using MoS2 nanotubes as potential oil additives." Wear 280 (2012): 36-45. | |
| [5] Kheireddin, Bassem. Tribological properties of nanoparticle-based lubrication systems. Diss. 2013. | |
| [6] Ghanbarzadeh, A., Wilson, M., Morina, A., Dowson, D., & Neville, A. (2016). Development of a new mechano-chemical model in boundary lubrication. Tribology International, 93, 573???582. | |
| [7] Gulzar, M., et al. "Tribological performance of nanoparticles as lubricating oil additives." Journal of Nanoparticle Research 18.8 (2016): 1-25. | |
| [8] Sui T, Song B, Zhang F, Yang Q (2015) Effect of particle size and ligand on the tribological properties of amino functionalized hairy silica nanoparticles as an additive to polyalphaolefin. J Nanomater 2015:9. | |
| [9] Aldana, P. U., Dassenoy, F., Vacher, B., Mogne, T. L., Thiebaut, B., & Bouffet, A. (2016). Antispalling Effect of WS2 Nanoparticles on the Lubrication of Automotive Gearboxes. Tribology Transactions, 59(1), 178???188. | |
| [10] Xu, Z. Y., Xu, Y., Hu, K. H., Xu, Y. F., & Hu, X. G. (2015). Formation and tribological properties of hollow sphere-like nano-MoS2 precipitated in TiO2 particles. Tribology International, 81, 139???148. doi:10.1016/j.triboint.2014.08.012 | |
| [11] Torres, CM Sotomayor, et al. "Nanoimprint lithography: an alternative nanofabrication approach." Materials Science and Engineering: C 23.1-2 (2003): 23-31. | |
| [12] Charitidis, Costas A., et al. "Manufacturing nanomaterials: from research to industry." Manufacturing Review 1 (2014): 11. | |
| [13] Gautam, Milan, Jong Oh Kim, and Chul Soon Yong. "Fabrication of aerosol-based nanoparticles and their applications in biomedical fields." Journal of Pharmaceutical Investigation (2021): 1-15. | |
| [14] Lu, Jing, et al. "Preparation of gas sensing CoTiO3 nanocrystallites using EDTA as the chelating agent in a sol???gel process." Ceramics International 41.3 (2015): 3714-3721. | |
| [15] Chen, Dong-Hwang, and Xin-Rong He. "Synthesis of nickel ferrite nanoparticles by sol-gel method." Materials Research Bulletin 36.7-8 (2001): 1369-1377. | |
| [16] Anand, Gautam, and Prateek Saxena. "A review on graphite and hybrid nano-materials as lubricant additives." IOP Conference Series: Materials Science and Engineering. Vol. 149. No. 1. IOP Publishing, 2016. | |
| [17] Hou, Xianjun, et al. "An experimental study and mechanism analysis on improving dispersion stability performance of Al2O3 nanoparticles in base synthetic oil under various mixing conditions." Journal of Nanoparticle Research 23.4 (2021): 1-16. | |
| [18] Zhao, Jun, et al. "Nanolubricant additives: A review." Friction 9.5 (2021): 891-917. | |
| [19] Tonk, Ravinder. "The challenges and benefits of using carbon nano-tubes as friction modifier lubricant additives." Materials Today: Proceedings 37 (2021): 3275-3278. | |
| [20] Sweeney, Scott F., Gerd H. Woehrle, and James E. Hutchison. "Rapid purification and size separation of gold nanoparticles via diafiltration." Journal of the American Chemical Society 128.10 (2006): 3190-3197. | |
| [21] Wright, Roger AE, et al. "Oil???soluble polymer brush grafted nanoparticles as effective lubricant additives for friction and wear reduction." Angewandte Chemie 128.30 (2016): 8798-8802. | |
| [22] Jost, H.P.. (2005). Tribology micro & macro economics: A road to economic savings. Lubrication Engineering. 61. 18-22. | |
| By Dr. Raj Shah, Mr. Blerim Gashi, Mr. Avishek Mojumdar, Dr. Steve Nitodas. The authors wish to thank Dr. George Diloyan for reviewing the manuscript. | |
|
Dr. Raj Shah is a Director at Koehler Instrument Company in New York, where he has worked for the last 27 years. He is an elected Fellow by his peers at IChemE, CMI, STLE, AIC, NLGI, INSTMC, Institute of Physics, The Energy Institute and The Royal Society of Chemistry. An ASTM Eagle award recipient, Dr. Shah recently coedited the bestseller, ???Fuels and Lubricants handbook???, details of which are available at ASTM???s Long-Awaited Fuels and Lubricants Handbook 2nd Edition Now Available (https://bit.ly/3u2e6GY). He earned his doctorate in Chemical Engineering from The Pennsylvania State University and is a Fellow from The Chartered Management Institute, London. Dr. Shah is also a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute and a Chartered Engineer with the Engineering council, UK. Dr. Shah was recently granted the honorific of ???Eminent engineer??? with Tau beta Pi, the largest engineering society in the USA. He is on the Advisory board of directors at Farmingdale university (Mechanical Technology ) , Auburn Univ ( Tribology ) and Stony Brook University ( Chemical engineering/ Material Science and engineering). An Adjunct Professor at the State University of New York, Stony Brook, in the Department of Material Science and Chemical engineering, Raj also has over 475 publications and has been active in the petroleum industry for over 3 decades. More information on Raj can be found at https://bit.ly/3sayVgT Mr. Blerim Gashi and Mr. Avishek Mojumdar are part of a thriving internship program at Koehler Instrument company and students of chemical engineering at State University of New York, Stony Brook, where Dr. Shah currently heads the External advisory board of directors. Dr. Steve (Stephanos) Nitodas is currently a member of the Faculty of the Department of Materials Science and Chemical Engineering at Stony Brook University, NY. His expertise lies in the synthesis and applications of nanostructured carbon and polymer nanocomposites. He has served as Coordinator/Principal Investigator in five (5) EU funded research projects of 4.2 million Euro total budget, and he has been involved as Co-Principal Scientist in thirteen (13) other funded research projects. Dr. Nitodas has worked for several years in the nanotechnology industry, possessing significant know-how related to transfer of knowledge from academia to the industry and the setup of startup companies. |
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
