| Feb 10, 2022 | |
Optimizing methods for stable storage of nucleic acid nanoparticles and their transportation at ambient temperatures |
|
| (Nanowerk Spotlight) Advances in nucleic acids research and therapeutic nanotechnology have allowed for the development of rationally designed therapeutic nucleic acids (TNAs) that have played a crucial role in many clinical applications. | |
| TNAs have become even more relevant due to the current COVID-19 pandemic as they were implemented in two FDA-approved mRNA-based nanoformulated vaccines. | |
| From the foundation and great promise laid by TNAs arose the development of novel nucleic acid nanoparticles (NANPs) – dynamic nanostructures with fine-tunable physichochemical properties and biological activities. | |
| NANPs have found success in a variety of biochemical applications ranging from nanoscaffolds for coordinated delivery of multiple TNAs to potent immunoregulators and biosensors. | |
| Despite their potential, the true viability of NANPs and TNAs is limited by their relative chemical instability and sensitivity to higher temperatures. Shipping and transportation of NANPs and TNAs currently relies on a cold-chain storage. This process can be costly and difficult to maintain in areas that lack needed resources. Breaking this chain can lead to major structural compromises which greatly reduce the functional effectiveness of the NANPs. | |
| Traditionally, in regular laboratory settings, nucleic acids have been dried using vacuum concentrators (SpeedVAc), which may also subject the samples to heat and infrared radiation to speed up the drying process. While it serves its purpose of removing the water content, the exposure to high temperature may alter architectural parameters or even damage the structures of the NANPs, an irreversible consequence, which renders them practically nonfunctional. | |
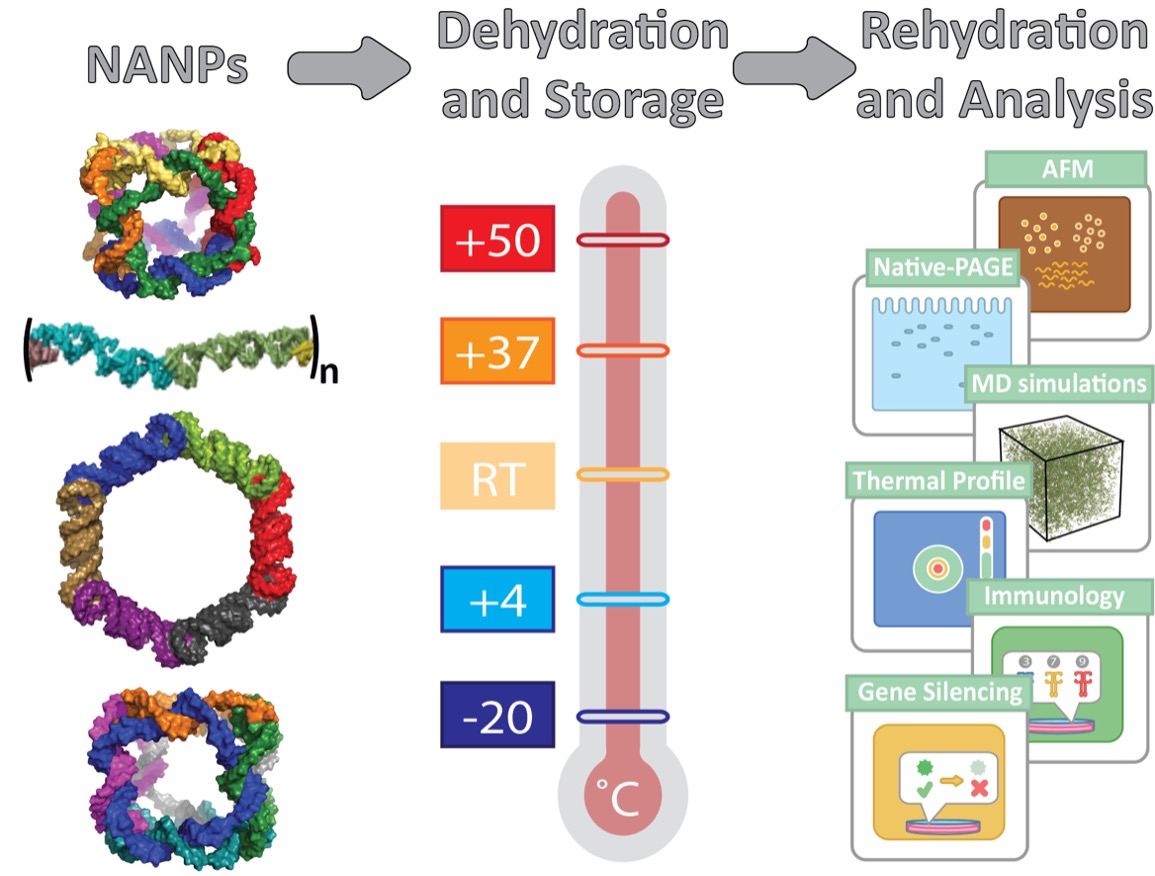
| In a paper in Small ("Anhydrous Nucleic Acid Nanoparticles for Storage and Handling at Broad Range of Temperatures"), an interdisciplinary team of scientists from The University of North Carolina at Charlotte, Penn State College of Medicine, and Frederick National Laboratory for Cancer Research sought to resolve this issue by exploring the feasibility of two additional approaches suitable for NANPs dehydration: lyophilization (lyo), and light-assisted drying (LAD). | |
| Instead of primarily using heat like SpeedVac, lyo uses sublimation of pre-frozen samples while LAD, a recently introduced technique, employs a FLIR laser to evaporate water off of sample droplets. | |
 |
|
| Schematic outline of experimental work. (Image courtesy of the researchers) | |
| These methods have been extensively tested on a set of representative NANPs that would address the variances in composition (DNA vs RNA), connectivity, and shapes, as well relative differences in thermodynamic and chemical stabilities. | |
| After dehydration, all NANPs were stored at temperatures ranging from -20 °C to +50 °C, which simulates realistic extreme conditions during NANPs transportation and handling. | |
| After storage, all NANPs were then rehydrated and subjected to various tests, which analyze structural and functional retention as well as immunological properties. | |
| This pioneering findings provide helpful insight on the specific advantages and disadvantages of each method for a particular type of NANP. | |
| This information can be utilized when determining the most optimal method needed to achieve different goals in nanomaterial preparation. For example, the dehydration via lyo and LAD both effectively reduce the amount of structural damage that NANPs experience at high temperatures of up to +50 °C. | |
| Compared to LAD, lyo has the advantage of allowing for a greater sample size, which can translate well into larger scale manufacturing. Meanwhile, LAD provides precise control over heating power and time as well as uniformity of heating for each sample. | |
| We also found that the addition of trehalose introduces structural stability to all NANPs while retaining their immunostimulatory and pre-programmed biological properties. In nature, trehalose has been used by many organisms ranging from plants to tardigrades, robust microscopic organisms which can survive in extreme environments. Its successful use in our experimental settings presents it as a gateway tool for diverse applications in nucleic acid preservation. | |
| In future studies we will seek to further refine the specificities of each drying method in order improve outcomes. It would be worthwhile to explore the use of trehalose as an accessory protectant during the lyo process. Lyo can be modified to involve freezing via a shell bath or immersion via liquid nitrogen. LAD can be better optimized for larger sample sizes for scale-up production. | |
| Additionally, in order to observe the immunological effects in preparation for therapeutic applications, it will be important to investigate the effects of these methods in vivo. | |
| The greatest challenge remains in obtaining consistency in results across a diverse and consistently growing repertoire of nanomaterials. Optimizations would need to be refined to cater to each intended use and to the unique properties of each nanoparticle. | |
| Clinically, lyo and LAD could be potentially applied as effective tools to prepare nucleic acid-based vaccines. Lyo is already well-established in the world of biochemical manufacturing, a factor which would ease its incorporation towards mass production of TNAs. | |
| The implementation of these methods could reduce the need for cold chain storage and dramatically improve vaccine shelf life. | |
| Overall, our work is valuable in developing user-friendly and affordable technologies for preparing nucleic acid nanoparticles. Refining these methods would allow for production of more durable nanomaterials that have high viability in withstanding environmental unpredictability during transportation. Bypassing these shipping and transportation barriers would provide broader access to a much-needed resource and pave the way for future applications in medicine and therapeutics. | |
| By Allison Tran and Kirill Afonin, Department of Chemistry, The University of North Carolina at Charlotte | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
