| May 30, 2022 | |
Nanomaterial-enabled bacteriophage therapy for wound infections |
|
| (Nanowerk Spotlight) Antimicrobial resistance (AMR) is a natural process that occurs when a strain of microbes (bacteria, viruses, fungi and parasites) develops a mechanism to thwart the effect of a drug. It then no longer responds to medicines, making the infections more difficult to treat and increasing the risk of spreading the disease. | |
| The World Health Organization (WHO) has published a list of bacteria for which new antibiotics are urgently needed, including Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacteriaceae, Enterococcus faecium, Staphylococcus aureus, and Salmonellae. | |
| According to the WHO, antibiotic resistance is one of the biggest threats to global health, food security, and development today: "Antibiotic resistance is rising to dangerously high levels in all parts of the world. New resistance mechanisms are emerging and spreading globally, threatening our ability to treat common infectious diseases. A growing list of infections – such as pneumonia, tuberculosis, blood poisoning, gonorrhea, and foodborne diseases – are becoming harder, and sometimes impossible, to treat as antibiotics become less effective." (check out the WHO's antimicrobial resistance fact sheet for more information). | |
| At the root of this problem are several factors: Easy access to antibiotics for human or animal use without a prescription; the over-prescription by health workers and veterinarians in countries without standard treatment guidelines; and the widespread use of antibiotics in agriculture and aquaculture for therapeutic, prophylactic and growth-promoting purposes (residual antibiotics remaining in the flesh at the time of slaughter may result in direct exposure of the consumer to these drugs). | |
| Additionally, the spontaneous evolution, bacterial mutation, and passing the resistant genes through horizontal gene transfer are significant contributors to antimicrobial resistance. | |
| Many studies have indicated the disastrous financial consequences of AMR including exorbitantly high healthcare costs due to an increase in hospital admissions and drug usage. Antibiotics are effective for killing off harmful bacteria – unfortunately they also kill off the good bacteria, which are necessary to maintain a healthy immune system. | |
| An estimated 1.27 million people died worldwide in 2019 from infections caused by drug-resistant bacteria, according to a landmark study published in The Lancet ("Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis"; PDF). This number is projected to reach 10 million by 2050 (Tackling Drug-resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance; PDF). | |
| The threat posed by antibiotic-resistant bacterial infections is exacerbated by the fact that traditional bacterial detection methods, including culturing, ELISA, and polymerase chain reaction (PCR) methods, suffer from inherent and significant shortcomings, such as very long processing times and the need for specialized reagents and equipment. | |
| Alternatives to antibiotics can be broadly define as any substance that can be substituted for therapeutic drugs that are becoming increasingly ineffective against pathogenic bacteria due to antimicrobial resistance. | |
| One interesting alternative to traditional antibiotics is phage therapy. Bacteriophages (derived from the Greek words meaning 'bacteria eater'), or just 'phages' for short, are viruses that selectively target and solely kill bacteria, even multi-drug resistant ones. Phages consist of Deoxyribonucleic acid (DNA) or Ribonucleic acid (RNA) enclosed within a protein capsid. | |
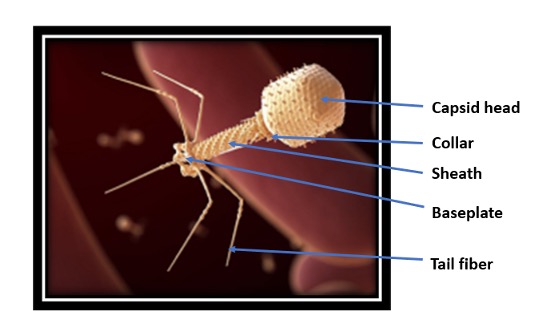 |
|
| Figure 1. An adapted computer-generated rendition of a bacteriophage. (Image: National Institute of Allergy and Infectious Disease, NIAID) | |
| Unlike traditional broad-spectrum antibiotics, phage therapy has several advantages: | |
|
|
|
| The unique selective and efficient targeting abilities of phages suggest that they could be applied to solve various problems in the area of bacterial detection and treatment of infection. | |
| Phages latch onto a bacteria's cell wall and then inject genetic material for replication, killing or at least slowing the growth of the bacteria. The viral genome effectively replaces the bacterial genome, halting the bacterial infection. The bacterial cell causing the infection is unable to reproduce, and instead produces additional phages (read more: "Fighting Fire with Fire: Killing bacteria with virus"). | |
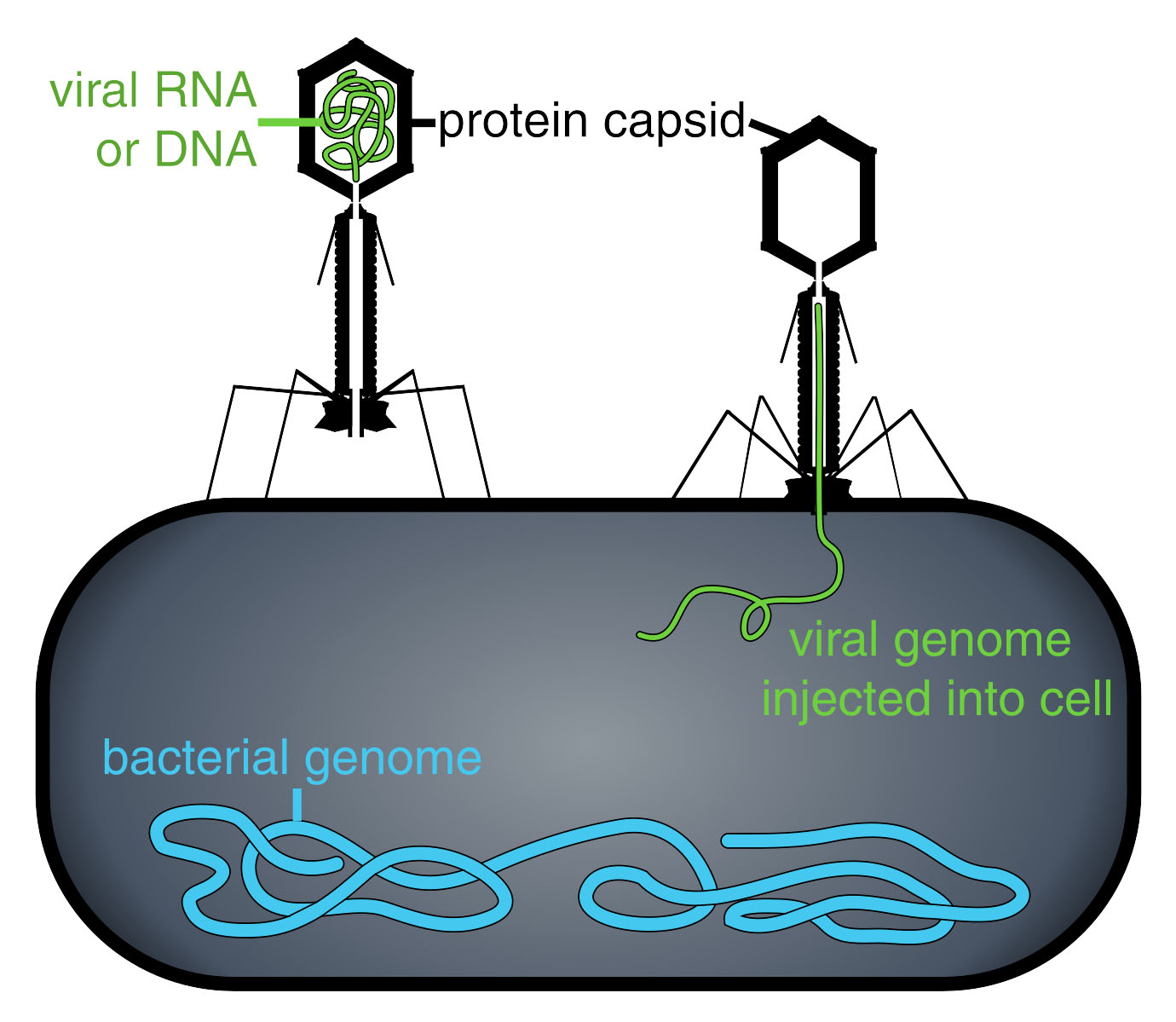 |
|
| Figure 2. Phage injecting its genome into bacteria. (Image: Thomas Splettstoesser (www.scistyle.com), CC BY-SA 3.0, via Wikimedia Commons) | |
| Despite their promising potential to fight bacterial infections, phages have not become a leading therapy to deal with antimicrobial resistant bacteria. Moreover, phage therapy has not received regulatory approval for human use (except for compassionate/emergency use and in the food sector). | |
| To date, phage therapy investigations have largely been observational or conducted in small, non-randomized trials. Consequently, scientists don't yet have the full picture of how it works and what the potential risks are. | |
| One obvious limitation to phage therapy is that phages are replicating and evolving entities whose biology is yet to be understood: There is a lack of rigorous biological characterization for most of the phage types, some of which may carry toxin genes. Some bacteriophages could also cause generalized transduction of bacterial genes, which could spread antibiotic resistance genes among pathogens. | |
| In general, the pharmacokinetics and pharmacodynamics of phages are difficult to model due to their exponential replication and rapid evolution – which presents a major barrier to their clinical translation. | |
| In view of these challenges, researchers have considered an alternative approach where phages are destroyed immediately after use, thus controlling dosage and circumventing undesirable consequences while maintaining the advantages of whole phage as antibacterial delivery vehicle. | |
| In 2019, a research team led by Professor Irene Chen of UCLA followed this novel approach to kill targeted bacterial cells using the photothermal effect (PNAS, "Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages"). The team accomplished this by conjugating phages to gold nanorods (which they termed 'phanorods'), whose excitation by near-infrared light causes localized heating that essentially cooks nearby bacteria. Thus, the phages deliver gold nanorods to the targeted bacteria, and the nanorods destroy both bacteria and phages simultaneously. This strategy transforms phages from an evolving biological entity into a controlled, drug-like reagent. | |
| In 2022, a joint international research team led by Chen, once again undertook bacteriophage therapy studies to heal the skin infection caused by antibiotic resistant Pseudomonas aeruginosa ("Treatment of Wound Infections in a Mouse Model Using Zn2+-Releasing Phage Bound to Gold Nanorods"). | |
| In this work, the team developed phage-nanomaterial conjugates that target the pathogen Pseudomonas aeruginosa and that can be used for the treatment of infected wounds. The team attached gold nanorods that can absorb near-infrared (NIR) light to P. aeruginosa-targeting phages. The phages were also decorated with a zinc-binding peptide. The release of Zn2+ promotes wound healing and inhibits bacterial growth. | |
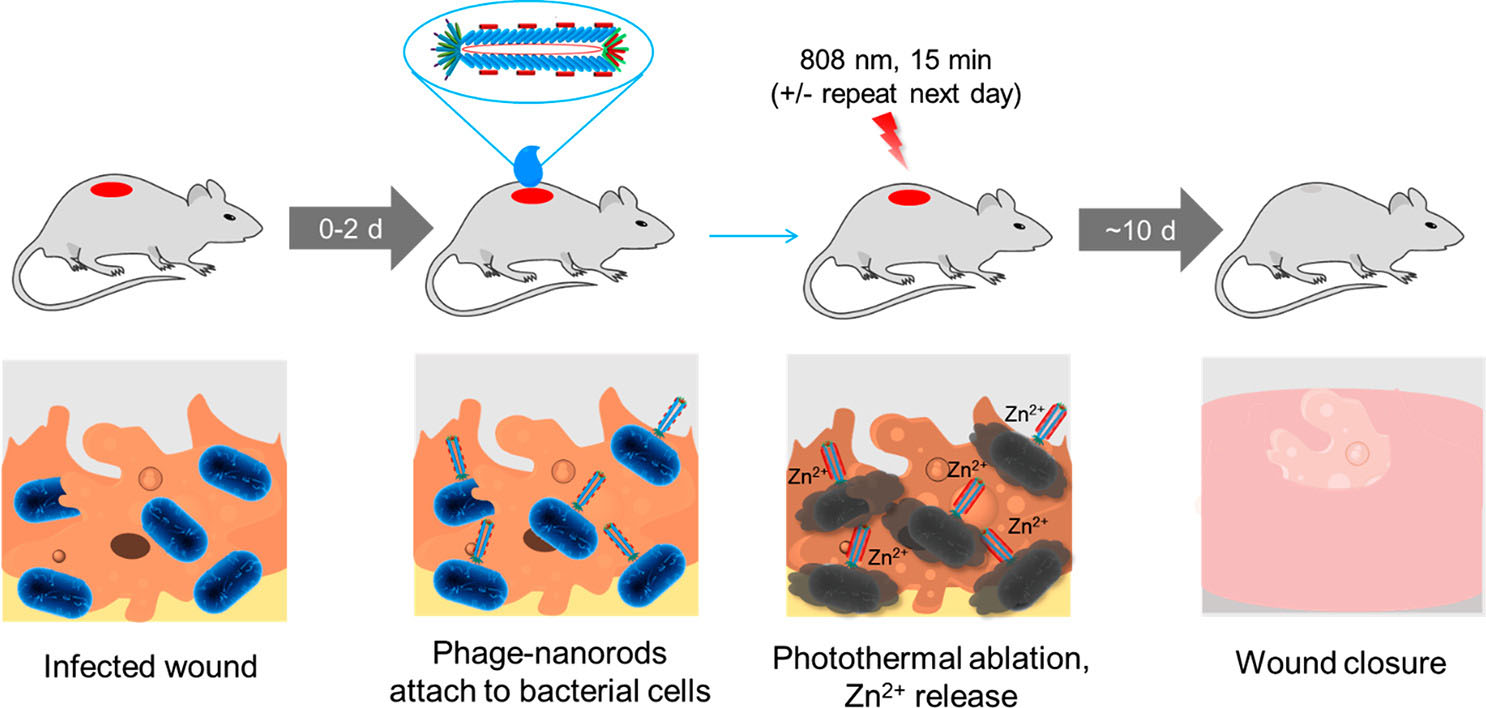 |
|
| Figure 3. Treatment of a P. aeruginosa-infected wound by phage-conjugated gold nanorods decorated with Zn2+. (Reprinted from doi:10.1021/acsnano.2c00048 with permission by American Chemical Society) | |
| As shown in the above figure, the treatment was initiated in a mouse model during the first two days after wound inoculation. The phage-based reagent was applied to the wound and allowed to attach for 30 minutes. The wound was irradiated with near-infrared light for 15 minutes to trigger photothermal ablation of P. aeruginosa and release of Zn2+; irradiation may be repeated the next day. Wound healing was observed over the next ∼10 days. | |
| The researchers found their treatment to be more effective than standard-of-care antibiotics, with a greater reduction in bacterial load and a faster reduction of the wound size. The phage-nanorod conjugates showed no detectable toxicity in mice. | |
| These recent results indicate that phage therapy controlled by inorganic nanomaterials has the potential to be a safe and effective antimicrobial strategy in vivo. | |
|
By Yashwant Mahajan, Associate Editor, Nanowerk
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
