| Posted: Feb 04, 2016 |
A fast solidification process makes material crackle
(Nanowerk News) What does it sound like when liquids solidify very fast?
|
|
Researchers from the Centre of Excellence in Computational Nanoscience at Aalto University and their colleagues at Brown University and the University of California, Irvine, have developed a theory that answers this question by combining for the first time the understanding of vibrations in solid material and the solidification of liquid at a microscopic level. The results were published in the renowned scientific publication Physical Review Letters ("Consistent Hydrodynamics for Phase Field Crystals").
|
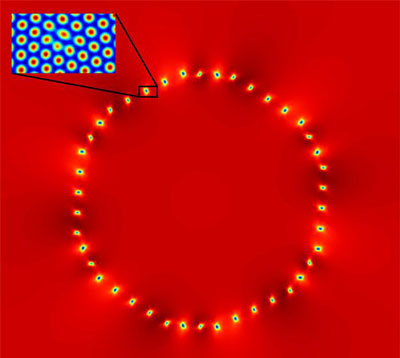 |
| The computational density field shows a circular solid crystal with a different orientation in relation to the surrounding solid material. The blue areas seen on the edges are lattice defects caused by differences in orientations. One of them has been magnified in order to show the defect in the periodic structure of the lattice. (Image: Aalto University)
|
|
'Earlier theories have focused on slow solidification. It is effectively diffusion in which the movement of atoms is slow and random. When solidification is fast, the atoms do not move only randomly, but the reaction is as if they had been compressed together. What our model can predict is the sound wave that is formed when the compression relaxes,' describes doctoral candidate Vili Heinonen.
|
|
Liquids usually solidify so slowly that no sound is created. When the process is fast, as it is when supercool water freezes, large defects are formed in the lattice structure of the material. According to Vili Heinonen, the wave formed when the defects relax resembles a kind of crackling. Effectively, the human ear cannot hear the sound, as supercool liquids that occur in nature are small droplets.
|
|
In addition to the crackling, the model also reveals a lot more.
|
|
'It helps us understand and predict the defects that are formed in materials - especially in metals - when they solidify. Also, materials often have different interfaces that are not always compatible. By understanding these defects we can also understand better why metals produced in certain temperatures have certain properties - and whether we can do something about it,' Heinonen explains.
|
|
The researchers proved the functionality of the model by analysing vibrations theoretically and computationally. In the computational test, the model was compared with predictions given by earlier theories, and it was demonstrated that the properties of the new theory are vitally important when we want to understand how, for example, the microscopic structure of metals changes in high temperatures. In addition to enabling control over the process of metal production, understanding these matters could in future enable a cheap method to produce intelligent nanostructures.
|

