| Dec 06, 2012 |
Individual cells respond to electrical signal
|
|
(Nanowerk News) Researchers at the Department of Molecular Nanofabrication (part of the University of Twente’s MESA+ Institute for Nanotechnology) have published their preliminary results in a prominent article in Angewandte Chemie ("A Supramolecular System for the Electrochemically Controlled Release of Cells").
|
|
This work was carried out in the context of a European Research Council (ERC) project that was awarded in 2010 for a period of five years. The leader of the research team, Dr Pascal Jonkheijm, explains that “Our approach to coupling chemistry at protein level worked so well that we immediately went one step further. Now we can use supramolecules to electrically switch the behaviour of individual cells. This occurs under the same physiological conditions as those found in the body.”
|
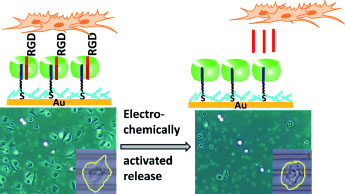 |
| Please release me: Electrochemically activated cell release is achieved using a redox-active supramolecular complex (see picture). Host molecule CB[8] (green) links surface-bound viologen (purple) with solution-exposed RGD peptides (red). Electrochemical reduction dissociates the complex, releases the peptides, and thus releases the cells from the substrates. This supramolecular strategy is also applicable to microelectrodes. (©Wiley)
|
|
The latter finding is enormously important in terms of the highly specific and local administration of medication, at the molecular level. The trick is to bind ligands (antibodies) to diseased cells. The success or failure of this approach is not simply a question of pure chemistry. It also depends on the, occasionally indefinable, “watery” conditions around the cell.
|
|
Dr Jonkheijm and the publishers of Angewandte Chemie are excited by this new method, because it enables ligands to be presented “dynamically”. An external electric field determines whether the cells bind to the ligands or unbind from them. The experiments carried out by these University of Twente researchers involved an order of magnitude of 0.4 volts. On a specially prepared surface, a “wound” inflicted on a cell-covered substrate healed significantly faster than it would under normal conditions in a healthy body.
|
|
The star of the show in this approach is a pumpkin-shaped macromolecule that can accommodate two linear “guest molecules” in its “skeleton”. One binds to a specially prepared gold surface, the other stretches its “feelers” out to a specific (diseased) body cell. The links appear to be reversible. Reversing the electrical signal causes cells to bind or to unbind. In some cases it is possible to partially bind the cells.
|
|
Towards applications
|
|
Pascal Jonkheijm feels that “This research opens the way to studies of fundamental aspects of cell biology. At the same time, however, together with researchers at the University of Twente’s MIRA Institute for Biomedical Technology and Technical Medicine and our partners in the BioMedical Materials programme, we are also considering possible applications. We want to attach the molecules to platforms carrying various ligands, which we can then present dynamically in terms of time and place. In this way, we can force or “trigger” target cells to bind under the most natural conditions. In the case of regeneration, for example, natural factors often play a decisive part. For instance, in the worst case, any infections that develop during treatment can lead to rejection. In such situations, the ability to control events at the cellular level is an important tool.”
|
|
The researchers (Dr Qi An and PhD students Jenny Brinkmann and Sven Krabbenborg) also want to see whether this method might be useful for detecting specific cells, using “cell fingerprints”.
|

