Showing Spotlights 121 - 128 of 151 in category All (newest first):
 Radioactive material is toxic because it creates ions when it reacts with biological molecules. These ions can form free radicals, which damage proteins, membranes, and nucleic acids. Free radicals damage components of the cells' membranes, proteins or genetic material by "oxidizing" them - the same chemical reaction that causes iron to rust. This is called 'oxidative stress'. Many forms of cancer are thought to be the result of reactions between free radicals and DNA, resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy. Nanotechnology has provided numerous constructs that reduce oxidative damage in engineering applications with great efficiency. As a new research report shows, nanotechnology applications could also help to remediate radioactive contamination at the source, by removing radioactive ions from the environment. Environmental contamination with radioactive ions that originate from the processing of uranium or the leakage of nuclear reactors is a potential serious health threat because it can leach into groundwater and contaminate drinking water supplies for large population areas. The key issue in developing technologies for the removal of radioactive ions from the environment and their subsequent safe disposal is to devise materials which are able to absorb radioactive ions irreversibly, selectively, efficiently, and in large quantities from contaminated water.
Radioactive material is toxic because it creates ions when it reacts with biological molecules. These ions can form free radicals, which damage proteins, membranes, and nucleic acids. Free radicals damage components of the cells' membranes, proteins or genetic material by "oxidizing" them - the same chemical reaction that causes iron to rust. This is called 'oxidative stress'. Many forms of cancer are thought to be the result of reactions between free radicals and DNA, resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy. Nanotechnology has provided numerous constructs that reduce oxidative damage in engineering applications with great efficiency. As a new research report shows, nanotechnology applications could also help to remediate radioactive contamination at the source, by removing radioactive ions from the environment. Environmental contamination with radioactive ions that originate from the processing of uranium or the leakage of nuclear reactors is a potential serious health threat because it can leach into groundwater and contaminate drinking water supplies for large population areas. The key issue in developing technologies for the removal of radioactive ions from the environment and their subsequent safe disposal is to devise materials which are able to absorb radioactive ions irreversibly, selectively, efficiently, and in large quantities from contaminated water.
Aug 13th, 2008
 Remember the movie blockbuster Erin Brockovich? The film is based on a real world legal case that revolves around hexavalent chromium, also known as chromium (VI), used by the Pacific Gas and Electric Company to control corrosion in cooling towers in its Hinkley, CA compressor station. Chromium (VI), a natural metal, is known to be toxic and is recognized as a human carcinogen via inhalation. It also is widely used by industry in the manufacture of stainless steel, welding, painting and pigment application, electroplating, and other surface coating processes. Researchers in Germany now have developed a novel method of multilayer anticorrosion protection including the surface pre-treatment by sonication and deposition of polyelectrolytes and inhibitors. This method results in the formation of a smart polymer nanonetwork for environmentally friendly organic inhibitors.
Remember the movie blockbuster Erin Brockovich? The film is based on a real world legal case that revolves around hexavalent chromium, also known as chromium (VI), used by the Pacific Gas and Electric Company to control corrosion in cooling towers in its Hinkley, CA compressor station. Chromium (VI), a natural metal, is known to be toxic and is recognized as a human carcinogen via inhalation. It also is widely used by industry in the manufacture of stainless steel, welding, painting and pigment application, electroplating, and other surface coating processes. Researchers in Germany now have developed a novel method of multilayer anticorrosion protection including the surface pre-treatment by sonication and deposition of polyelectrolytes and inhibitors. This method results in the formation of a smart polymer nanonetwork for environmentally friendly organic inhibitors.
Jul 29th, 2008
 Olive oil is good for your health. Unfortunately, the production of olive oil is not. During the olive oil production process, olive oil mills produce a liquid waste called olive black water or olive-oil-mill wastewater (OMW). This waste water has significant polluting properties due to its high levels of chemical oxygen demand (COD), biochemical oxygen demand (BOD), and phenols. In Mediterranean countries, which account for approximately 95% of the worldwide olive oil production, the annual amount of OMW is estimated to be over 30 million cubic meters. Disposal of OMV has therefore always been an important issue in this region especially since conventional wastewater treatment methods are relatively ineffective for removing the kind of pollutants found in OMW. On the other hand, OMW may also be regarded as an inexpensive biomass source of inorganic and organic compounds. Using suitable separation processes, these compounds can be recovered and transformed into products for use in agriculture, environmental biotechnology processes, and industry. New research has now investigated the interaction of complexing dissolved organic matter with aluminum oxide nanoparticles.
Olive oil is good for your health. Unfortunately, the production of olive oil is not. During the olive oil production process, olive oil mills produce a liquid waste called olive black water or olive-oil-mill wastewater (OMW). This waste water has significant polluting properties due to its high levels of chemical oxygen demand (COD), biochemical oxygen demand (BOD), and phenols. In Mediterranean countries, which account for approximately 95% of the worldwide olive oil production, the annual amount of OMW is estimated to be over 30 million cubic meters. Disposal of OMV has therefore always been an important issue in this region especially since conventional wastewater treatment methods are relatively ineffective for removing the kind of pollutants found in OMW. On the other hand, OMW may also be regarded as an inexpensive biomass source of inorganic and organic compounds. Using suitable separation processes, these compounds can be recovered and transformed into products for use in agriculture, environmental biotechnology processes, and industry. New research has now investigated the interaction of complexing dissolved organic matter with aluminum oxide nanoparticles.
Jun 13th, 2008
 Advanced material engineering techniques can structure surfaces that allow dynamic tuning of their wettability all the way from superhydrophobic (i.e. repelling) behavior to almost complete wetting (i.e. superhydrophilic or strongly absorbing) - but these surfaces only work with high-surface-tension liquids. Almost all organic liquids that are ubiquitous in human environment such as oils, solvents, detergents, etc. have fairly low surface tensions and thus readily wet even superhydrophobic surfaces. In a previous Spotlight we reported how researchers have been creating surfaces that would extend superhydrophobic behavior to all liquids, no matter what the surface tension. New work coming out of MIT now describes a novel nanostructures membrane that can be switched between superhydrophilic and superhydrophobic behavior on demand. Think of it as an 'oil spill clean-up paper towel' that absorbs only the oil but not the water. Given the global scale of severe water pollution arising from oil spills and industrial organic pollutants, this nanomaterial may prove particularly useful in the design of recyclable absorbents with significant environmental impact.
Advanced material engineering techniques can structure surfaces that allow dynamic tuning of their wettability all the way from superhydrophobic (i.e. repelling) behavior to almost complete wetting (i.e. superhydrophilic or strongly absorbing) - but these surfaces only work with high-surface-tension liquids. Almost all organic liquids that are ubiquitous in human environment such as oils, solvents, detergents, etc. have fairly low surface tensions and thus readily wet even superhydrophobic surfaces. In a previous Spotlight we reported how researchers have been creating surfaces that would extend superhydrophobic behavior to all liquids, no matter what the surface tension. New work coming out of MIT now describes a novel nanostructures membrane that can be switched between superhydrophilic and superhydrophobic behavior on demand. Think of it as an 'oil spill clean-up paper towel' that absorbs only the oil but not the water. Given the global scale of severe water pollution arising from oil spills and industrial organic pollutants, this nanomaterial may prove particularly useful in the design of recyclable absorbents with significant environmental impact.
May 30th, 2008
 It wasn't market forces that landed a man on the moon; and It wasn't market forces that led France to build a nuclear energy infrastructure that now enables it to generate some 75% of its entire energy needs from nuclear power (just an example of what energy policy can do; let's not get into a discussion here of nuclear energy, though). But somehow, the leading political and industrial forces in the United States - together with China the largest emitter of greenhouse gases on the planet - think that a task so fundamental and massive as fighting global warming and environmental pollution should mostly be left 'to the market'. Unfortunately, it's just a matter of economic reality that 'the market' will not invest in new energy technologies on a large scale until existing ways of producing energy become more expensive than producing alternative energies - which at the moment they aren't. As is the case with almost all emerging technologies, government initially lends a helping hand before the technology becomes a viable commercial proposition and the market takes over (remember how the Internet got created?). In the case of future clean energy technologies, it appears that this 'helping hand' needs to be massive and swift. It's not so much that clean/green tech wouldn't develop over time on its own. But it's against the backdrop of accelerating global warming that it becomes a top priority that requires massive public resources.
It wasn't market forces that landed a man on the moon; and It wasn't market forces that led France to build a nuclear energy infrastructure that now enables it to generate some 75% of its entire energy needs from nuclear power (just an example of what energy policy can do; let's not get into a discussion here of nuclear energy, though). But somehow, the leading political and industrial forces in the United States - together with China the largest emitter of greenhouse gases on the planet - think that a task so fundamental and massive as fighting global warming and environmental pollution should mostly be left 'to the market'. Unfortunately, it's just a matter of economic reality that 'the market' will not invest in new energy technologies on a large scale until existing ways of producing energy become more expensive than producing alternative energies - which at the moment they aren't. As is the case with almost all emerging technologies, government initially lends a helping hand before the technology becomes a viable commercial proposition and the market takes over (remember how the Internet got created?). In the case of future clean energy technologies, it appears that this 'helping hand' needs to be massive and swift. It's not so much that clean/green tech wouldn't develop over time on its own. But it's against the backdrop of accelerating global warming that it becomes a top priority that requires massive public resources.
Apr 30th, 2008
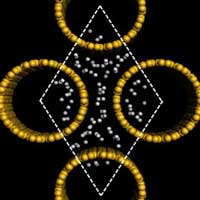 Safe, efficient and compact hydrogen storage is a major challenge in order to realize hydrogen powered transport. According to the U.S. Department of Energy's Freedom CAR program roadmap, the on-board hydrogen storage system should provide a gravimetric density of 6 wt% at room temperature to be considered for technological implementation. Currently, the storage of hydrogen in the absorbed form is considered as the most appropriate way to solve this problem. Research groups worldwide are seeking and experimenting with materials capable of absorbing and releasing large quantities of hydrogen easily, reliably, and safely. One candidate material that is being considered as a candidate for hydrogen storage media is single-walled carbon nanotubes. So far, carbon nanotubes have been unable to meet the DOE's hydrogen storage target. New theoretical work from China suggests that silicon nanotubes can store hydrogen more efficiently than their carbon nanotube counterparts. This raises the possibility that, after powering the micro-electronics revolution, silicon could also become a key material for the future hydrogen economy.
Safe, efficient and compact hydrogen storage is a major challenge in order to realize hydrogen powered transport. According to the U.S. Department of Energy's Freedom CAR program roadmap, the on-board hydrogen storage system should provide a gravimetric density of 6 wt% at room temperature to be considered for technological implementation. Currently, the storage of hydrogen in the absorbed form is considered as the most appropriate way to solve this problem. Research groups worldwide are seeking and experimenting with materials capable of absorbing and releasing large quantities of hydrogen easily, reliably, and safely. One candidate material that is being considered as a candidate for hydrogen storage media is single-walled carbon nanotubes. So far, carbon nanotubes have been unable to meet the DOE's hydrogen storage target. New theoretical work from China suggests that silicon nanotubes can store hydrogen more efficiently than their carbon nanotube counterparts. This raises the possibility that, after powering the micro-electronics revolution, silicon could also become a key material for the future hydrogen economy.
Apr 21st, 2008
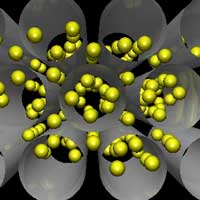 The greenhouse effect is primarily a function of the concentration of water vapor, carbon dioxide, and other trace gases in the Earth's atmosphere that absorb the terrestrial radiation leaving the surface of the Earth. Changes in the atmospheric concentrations of these greenhouse gases can alter the balance of energy transfers between the atmosphere, space, land, and the oceans. The capture and storage of greenhouse gases could play a significant role in reducing the release of greenhouse gases into the atmosphere (read more about capture and storage of carbon dioxide here). Carbon dioxide (CO2) is the most important greenhouse gas and captures the limelight in most reports on global warming. While other greenhouse gases make up less of the atmosphere, they account for about 40 percent of the greenhouse gas radiation sent back to Earth. They can also be much more efficient at absorbing and re-emitting radiation than carbon dioxide, so they are small but important elements in the equation. In fact, molecule-for-molecule some gases containing lots of fluorine are 10,000 times stronger at absorbing radiation than carbon dioxide. A new systematic computational study shows an interesting approach of how nanotechnology, in this case the use of carbon nanotubes and other nanomaterials, could lead to effective filters for the capture and storage of greenhouse gases.
The greenhouse effect is primarily a function of the concentration of water vapor, carbon dioxide, and other trace gases in the Earth's atmosphere that absorb the terrestrial radiation leaving the surface of the Earth. Changes in the atmospheric concentrations of these greenhouse gases can alter the balance of energy transfers between the atmosphere, space, land, and the oceans. The capture and storage of greenhouse gases could play a significant role in reducing the release of greenhouse gases into the atmosphere (read more about capture and storage of carbon dioxide here). Carbon dioxide (CO2) is the most important greenhouse gas and captures the limelight in most reports on global warming. While other greenhouse gases make up less of the atmosphere, they account for about 40 percent of the greenhouse gas radiation sent back to Earth. They can also be much more efficient at absorbing and re-emitting radiation than carbon dioxide, so they are small but important elements in the equation. In fact, molecule-for-molecule some gases containing lots of fluorine are 10,000 times stronger at absorbing radiation than carbon dioxide. A new systematic computational study shows an interesting approach of how nanotechnology, in this case the use of carbon nanotubes and other nanomaterials, could lead to effective filters for the capture and storage of greenhouse gases.
Apr 4th, 2008
 You probably have seen quite a number of research reports on the amazing climbing abilities of geckos. Here at Nanowerk, we ran several Spotlights on this topic, for instance on mimicking gecko toe structures to fabricate super-strong dry adhesives. One demonstration of so-called 'gecko tape' has already been used in building Stickybot, a quadruped robot capable of climbing smooth vertical surfaces, such as glass, acrylic and whiteboard. In addition to the animal kingdom, scientists have started looking at plants to identify biological climbing mechanisms that could be exploited for engineering applications. One obvious candidate is ivy, a climbing woody plant. Researchers now have found that ivy secretes nanoparticles which allow the plant to affix to a surface and play an important role in the plant's climbing capability. This ivy secretion mechanism may inspire new, 'green' methods for synthesizing nanoparticles biologically or new approaches to adhesion mechanisms for mechanical devices.
You probably have seen quite a number of research reports on the amazing climbing abilities of geckos. Here at Nanowerk, we ran several Spotlights on this topic, for instance on mimicking gecko toe structures to fabricate super-strong dry adhesives. One demonstration of so-called 'gecko tape' has already been used in building Stickybot, a quadruped robot capable of climbing smooth vertical surfaces, such as glass, acrylic and whiteboard. In addition to the animal kingdom, scientists have started looking at plants to identify biological climbing mechanisms that could be exploited for engineering applications. One obvious candidate is ivy, a climbing woody plant. Researchers now have found that ivy secretes nanoparticles which allow the plant to affix to a surface and play an important role in the plant's climbing capability. This ivy secretion mechanism may inspire new, 'green' methods for synthesizing nanoparticles biologically or new approaches to adhesion mechanisms for mechanical devices.
Mar 31st, 2008
 Radioactive material is toxic because it creates ions when it reacts with biological molecules. These ions can form free radicals, which damage proteins, membranes, and nucleic acids. Free radicals damage components of the cells' membranes, proteins or genetic material by "oxidizing" them - the same chemical reaction that causes iron to rust. This is called 'oxidative stress'. Many forms of cancer are thought to be the result of reactions between free radicals and DNA, resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy. Nanotechnology has provided numerous constructs that reduce oxidative damage in engineering applications with great efficiency. As a new research report shows, nanotechnology applications could also help to remediate radioactive contamination at the source, by removing radioactive ions from the environment. Environmental contamination with radioactive ions that originate from the processing of uranium or the leakage of nuclear reactors is a potential serious health threat because it can leach into groundwater and contaminate drinking water supplies for large population areas. The key issue in developing technologies for the removal of radioactive ions from the environment and their subsequent safe disposal is to devise materials which are able to absorb radioactive ions irreversibly, selectively, efficiently, and in large quantities from contaminated water.
Radioactive material is toxic because it creates ions when it reacts with biological molecules. These ions can form free radicals, which damage proteins, membranes, and nucleic acids. Free radicals damage components of the cells' membranes, proteins or genetic material by "oxidizing" them - the same chemical reaction that causes iron to rust. This is called 'oxidative stress'. Many forms of cancer are thought to be the result of reactions between free radicals and DNA, resulting in mutations that can adversely affect the cell cycle and potentially lead to malignancy. Nanotechnology has provided numerous constructs that reduce oxidative damage in engineering applications with great efficiency. As a new research report shows, nanotechnology applications could also help to remediate radioactive contamination at the source, by removing radioactive ions from the environment. Environmental contamination with radioactive ions that originate from the processing of uranium or the leakage of nuclear reactors is a potential serious health threat because it can leach into groundwater and contaminate drinking water supplies for large population areas. The key issue in developing technologies for the removal of radioactive ions from the environment and their subsequent safe disposal is to devise materials which are able to absorb radioactive ions irreversibly, selectively, efficiently, and in large quantities from contaminated water.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





