| Posted: Jul 29, 2008 | |
Self-healing nanotechnology anticorrosion coatings as alternative to toxic chromium |
|
| (Nanowerk Spotlight) Remember the movie blockbuster Erin Brockovich? The film is based on a real world legal case that revolves around hexavalent chromium, also known as chromium (VI), used by the Pacific Gas and Electric Company (PG&E) to control corrosion in cooling towers in its Hinkley, CA compressor station. Chromium (VI), a natural metal, is known to be toxic and is recognized as a human carcinogen via inhalation. It also is widely used by industry in the manufacture of stainless steel, welding, painting and pigment application, electroplating, and other surface coating processes. PG&E for instance would periodically treat the surface of the cooling coils in its Hinkley station with anti-corrosion paint and release the chromium-containing wastewater into the environment. | |
| The huge economic impact of the corrosion of metallic structures is a very important issue for all modern societies. Estimates for the cost of corrosion degradation run to about €200 billion a year in Europe and over $270 billion a year in the U.S. The annual cost of corrosion consists of both direct costs and indirect costs. The direct costs are related to the costs of design, manufacturing, and construction in order to provide corrosion protection, and the indirect costs are concerned with corrosion-related inspection, maintenance and repairs. | |
| In spite of its toxicity, chromium (VI) has remained an essential ingredient in the metal finishing industry for corrosion control. But combine the economic impact of corrosion damage, the environmental and health problems cause by chromium (VI), and the increasing regulatory restrictions, scientists have a huge incentive to develop a new generation of protective coating systems. | |
| The advanced materials that are being developed and used in modern industries require increasingly sophisticated coatings for improved performance and durability. With a degrading environment due to industrial factors, environmental compatibility is an aspect that gains in importance during the design phase of novel materials – and chromium (VI) compounds certainly wouldn't make the list. Furthermore, while conventional anticorrosion coatings are just passive barriers that prevent the interaction of corrosive species with a metal, future nanotechnology based protective coatings will be 'smart', i.e. they will provide several functionalities that will in effect result in self-healing capabilities. | |
| The whole concept of 'smart' materials that react on external impact (pH, humidity changes, or distortion of the coating integrity) and repair themselves has experienced a tremendous boost with the advent of nanotechnology. The nanoscale multilayer structure of a coating, in which the components are integrated and mutually reactive, is a main point in sophisticated and strong corrosion protection. | |
| Researchers in Germany now have developed a novel method of multilayer anticorrosion protection including the surface pre-treatment by sonication and deposition of polyelectrolytes and inhibitors. This method results in the formation of a smart polymer nanonetwork for environmentally friendly organic inhibitors. | |
| "Our novel coating exhibits very high resistance to corrosion attack, long term stability in aggressive media and an environmentally friendly, easy and economical preparation procedure," Dr. Daria Andreeva tells Nanowerk. "We have demonstrated the general procedure for a surface important for the aircraft industry but it is similarly applicable for many types of surfaces, thus enabling many applications in advanced technologies." | |
| Andreeva is a researcher at the Max Planck Institute of Colloids and Interfaces in Potsdam, Germany. Together with her colleagues Dmitri Fix, Dr. Dmitry Shchukin and Dr. Helmuth Möhwald, she published a paper on the design of the group's novel anticorrosion system in Advanced Materials ("Self-Healing Anticorrosion Coatings Based on pH-Sensitive Polyelectrolyte/Inhibitor Sandwichlike Nanostructures"). | |
 |
|
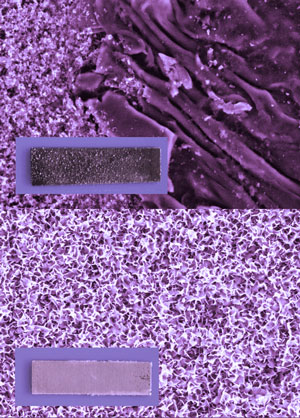
| 21-day-corrosion test in 0.1M NaCl solution: scanning electron microscopy image and photograph of uncovered aluminum plate with corrosion degradation (above) and corrosion resistant aluminum plate covered by polyelectrolyte coating (below). (Image: Dr. Andreeva, Max Planck Institute of Colloids and Interfaces) | |
| The main novelty of the proposed system is the multi-level protection approach, where the protective systems – the 'smart' multilayers – will not only be a barrier to external impacts, but also respond to changes in their internal structure, and combine in the same system different damage prevention and reparation mechanisms. | |
| The Max-Planck scientists started with the assumption that the layer-by-layer (LbL) deposition procedure would be a very effective solution for the preparation of self-healing anticorrosion coatings. The LbL process involves the stepwise electrostatic assembly of oppositely charged species (e.g., polyelectrolytes and inhibitors or nanoparticles) on a substrate surface with nanometer-scale precision, and allows the formation of a coating with multiple functionality. | |
| A novel step in this anticorrosion system is the surface pretreatment of aluminum alloy by intensive sonication in water with an ultrasonic horn. Although the typical aluminum surface is covered by a 3-7 nm thick natural oxide film, this thin layer is not sufficient to protect against corrosion agents and does not yield good adhesion to subsequent layers of the coating. | |
| "The ultrasonic pretreatment is crucial for formation of a uniform film" says Andreeva. "The surface of ultrasonically pretreated samples exhibits better wettability, adhesion, and chemical bonding with the polymer layers of the subsequent LbL coating. It results in a homogeneous distribution of the polymer film on the aluminum surface." | |
| After pretreatment, 5-10 nm thick layers of polyelectrolytes and inhibitor were formed by LbL deposition on the freshly sonicated aluminum alloys. | |
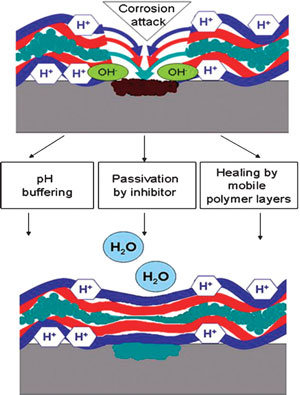
| The scientists were amazed that even the nanometer-thick polyelectrolyte/ inhibitor coating provides effective corrosion protection for the aluminum alloy. They explain that the nature and properties of their novel anticorrosion coating simultaneously provide three mechanisms of corrosion protection: 1) passivation of the metal degradation by controlled release of inhibitor; 2) buffering of pH changes at the corrosive area by polyelectrolyte layers; and 3) self-curing of the film defects owing to the mobility of the polyelectrolyte constituents in the layer-by-layer assembly. | |
 |
|
| Schematic mechanism of corrosion protection. (Reprinted with permission from Wiley) | |
| Since the release of the inhibitor is stimulated by corrosive species and corrosion products, this 'smart' coating enables prolongated self-healing activity. | |
| This anticorrosion protection method has a very broad range of potential applications due to its versatility. All components (polyelectrolytes and inhibitors) could be adjusted for a particular surface. The novel coating could be applied in aerospace, automotive and maritime industry and all other areas that suffer from corrosion damage, such as for instance oil and gas pipelines. | |
| "Although we concentrated on corrosion, our method could also be more generally applied for self-repairing coatings like antifungal or antifriction applications" Andreeva points out. | |
| One of the practical problems the Max-Planck team is still working on is the automation of the layer formation procedure in order to allow the scaling up of their technique for industrial applications. Beyond that, they are already looking to introducing other components with other mechanisms of corrosion protection into the system such as the combination of several inhibitors or self-polymerized compounds. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
