AES (Auger Electron Spectroscopy)
Definition: Auger Electron Spectroscopy (AES) is a powerful surface analysis technique that reveals the elemental composition of the top few atomic layers of a material
Principle of Operation
AES operates on the principle of the Auger effect, named after the French physicist Pierre Auger. When a material's surface is bombarded with a beam of high-energy electrons or photons, it causes the ejection of core electrons from the atoms on the surface. This creates vacancies, leading to a cascade of electron transitions within the atom as electrons from higher energy levels drop down to fill the vacancy. The energy released during these transitions can cause the emission of another electron, known as the Auger electron. In the Auger process, the energy of these Auger electrons is characteristic of the atomic structure of the element from which they are emitted, allowing for the precise identification of the elemental composition of the surface being analyzed.
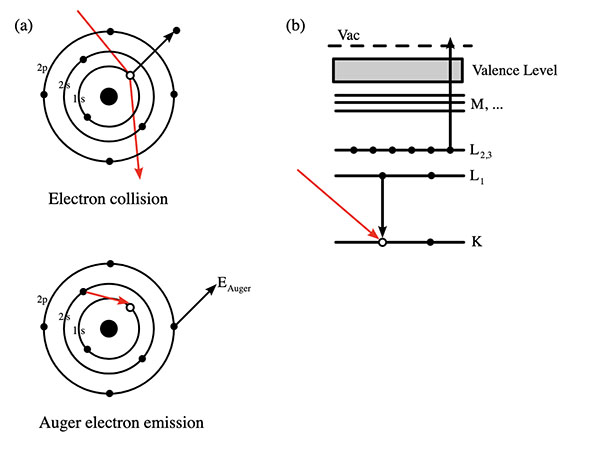
Key Features of the Auger Process
- Surface Sensitivity: AES is extremely sensitive to the surface layers of materials, typically analyzing the top 2 to 5 nanometers. This makes it an invaluable tool for studying thin films, surface treatments, and interfaces.
- Elemental Analysis: It can detect and quantify nearly all elements from the periodic table, except for hydrogen and helium, providing detailed information about the surface composition of the material under study.
- Spatial Resolution: Modern AES instruments, equipped with focused electron beams, can achieve spatial resolutions of a few nanometers, enabling the detailed mapping of elemental distributions across small areas of the surface.
- Quantitative Analysis: By measuring the intensity of the Auger peaks, it is possible to quantify the concentration of elements present on the surface, although this requires careful calibration and consideration of matrix effects.
Limitations
While AES provides detailed surface compositional analysis, it does have some limitations:
- Damage to Sensitive Samples: The high-energy electron beam can potentially damage or alter the surface of sensitive materials.
- Vacuum Requirement: AES requires a high-vacuum environment to prevent scattering of electrons by air molecules, which can limit the analysis of volatile or non-vacuum compatible materials.
- Complex Data Interpretation: The interpretation of AES spectra can be complex, requiring a thorough understanding of the Auger process and the potential for peak overlaps, especially in materials with a complex composition.
Applications
AES is widely used across various fields of research and industry, including:
- Materials Science: For investigating the surface composition and corrosion properties of metals and alloys.
- Semiconductor Manufacturing: In the analysis of thin films, contamination, and interface quality, critical for device fabrication.
- Catalysis: To study the surface composition of catalysts and their interaction with reactants.
- Surface Coatings and Treatments: For characterizing the composition and thickness of coatings, as well as the effectiveness of surface treatments.
Comparison to XPS
Auger Electron Spectroscopy (AES) and X-ray Photoelectron Spectroscopy (XPS) are both essential for analyzing material surfaces, offering insights into elemental compositions and chemical states. Both techniques probe the top few nanometers of surfaces, crucial for studying coatings, thin films, and surface contaminants. However, they differ significantly in their approach and the details they reveal. AES utilizes an electron beam to initiate the Auger effect, which results in the emission of secondary electrons specific to the elements present. This makes AES excellent for high-resolution imaging and pinpoint analysis of surface features.
Conversely, XPS involves irradiating the sample with X-rays to eject core electrons, with the emitted electrons' binding energies providing elemental and chemical state information. XPS can almost detect all elements, offering quantitative analysis and the ability to identify chemical states through binding energy shifts. While AES excels in spatial resolution, allowing for detailed surface mappings, XPS is superior in quantifying surface compositions and understanding chemical environments. Thus, although AES and XPS share a focus on surface analysis, they serve complementary roles: AES for spatial detail and XPS for chemical specificity.
Further Reading
The European Physical Journal Plus, Auger spectroscopy beyond the ultra-short core-hole relaxation time approximation
