X-ray Photoelectron Spectroscopy (XPS)
Definition: X-ray Photoelectron Spectroscopy (XPS) is a surface analysis technique that measures the elemental composition, empirical formula, chemical state, and electronic state of the elements within a material. XPS is also commonly referred to as Electron Spectroscopy for Chemical Analysis (ESCA), highlighting its capability in determining chemical environments.
Principle of Operation
XPS relies on the photoelectric effect, where irradiating a material with X-rays causes the emission of core electrons. The kinetic energy of these emitted electrons is measured, which can be directly related to the binding energy of the electrons within the atoms. This allows for the identification of the elements present, their chemical state, and the overall electronic structure of the surface layer. The process begins with the absorption of X-ray photons by atoms in the material, leading to the ejection of photoelectrons. By analyzing the energy spectrum of these photoelectrons, information about the surface composition and chemical states of the elements can be deduced.
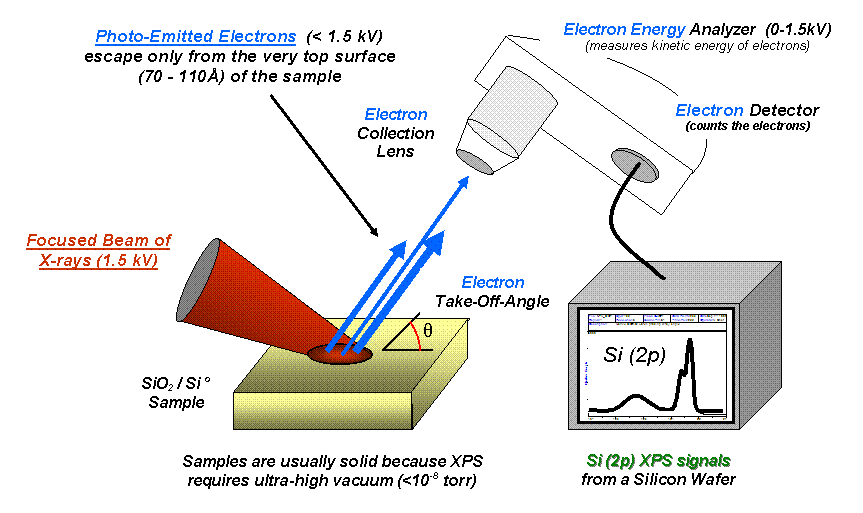
Key Features of XPS
- Chemical State Information: XPS provides insights into the chemical states of the elements, allowing for the differentiation between oxidation states and compounds.
- Elemental Composition: It can detect all elements with atomic numbers from beryllium (Be) onwards, offering a comprehensive overview of the surface composition.
- Depth Profiling: Through angle-resolved XPS or sputtering, it can provide information about the depth distribution of elements, enabling the analysis of multi-layer structures.
- Quantitative Analysis: XPS can quantify the composition of the elements present on the surface based on the intensity of the photoelectron peaks, with sensitivity to the environment around the atoms.
Limitations
Despite its comprehensive analysis capabilities, XPS has some limitations:
- Sensitivity to Surface Contamination: Being a surface-sensitive technique, XPS can be significantly affected by surface contamination.
- Vacuum Requirement: The technique requires ultra-high vacuum conditions to prevent the scattering and absorption of photoelectrons by air.
- Sampling Depth: XPS analyzes only the top 1-10 nm of the material surface, which may not be representative of the bulk material.
Applications
XPS is utilized in a wide range of fields for surface chemical analysis, including:
- Electronics: XPS is crucial in the study of semiconductor interfaces, especially in devices like metal-oxide-semiconductor field-effect transistors (MOSFETs). It is used to analyze the composition and electronic structure of the silicon dioxide (SiO2) interface with silicon (Si), which is essential for device performance. XPS can provide insights into the presence of contaminants, oxide thickness, and the chemical state of silicon, which directly impact the electrical properties and reliability of the semiconductor device
- Catalysis: XPS is widely used to study the surface chemistry of metal oxide catalysts, such as titanium dioxide (TiO2) and zinc oxide (ZnO), which are employed in photocatalysis and environmental cleanup processes. XPS can determine the oxidation state of the metal, the presence of defects, and the distribution of active sites, which are crucial factors influencing the catalyst's performance and stability.
- Materials Science: For characterizing the surface chemistry of materials and studying surface modifications.
- Corrosion Science: To investigate oxidation states and corrosion products on metal surfaces.
- Semiconductor Industry: In the analysis of thin films, interfaces, and contamination on semiconductor wafers.
- Polymer Science: To analyze the surface composition and treatments of polymers.
Comparison to AES
X-ray Photoelectron Spectroscopy (XPS) and Auger Electron Spectroscopy (AES) both provide valuable surface analysis capabilities, but with distinct focuses. XPS offers detailed chemical state information and a broader elemental detection range, making it invaluable for understanding chemical environments and reactions on surfaces. AES, with its high spatial resolution, is better suited for examining surface morphologies and distributions of elements with nanometer precision. Together, they offer complementary insights into material surfaces, with XPS excelling in chemical analysis and AES in spatial mapping.
Further Reading
Surface and Interface Analysis, The quantitative analysis of surfaces by XPS: A review
