Understanding Single Atom Catalysts
Definition: Single-atom catalysts (SACs) represent a novel class of catalysts that consist of isolated individual atoms anchored on a support material. These catalysts exhibit unique properties due to their maximal atom utilization and distinct electronic structure, leading to exceptional catalytic efficiency and selectivity. SACs hold the key to reducing pollution in chemical manufacturing
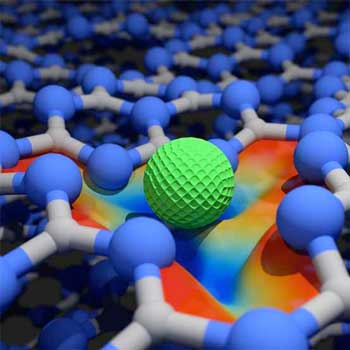
Definition and Principle of Operation
Single-atom catalysts are at the cutting edge of catalysis research, offering a blend of high efficiency, selectivity, and sustainability. Single-atom catalysts are defined by their structure where single atoms are dispersed on a suitable substrate, maximizing the exposure of active sites. Unlike nanoparticles or bulk materials, SACs offer unique active sites that are individually accessible, leading to unparalleled activity and specificity in chemical reactions.
By enabling precise control at the atomic level, SACs hold the promise of revolutionizing chemical processes across industries, from environmental remediation to energy production and beyond.
How Single-Atom Catalysts Work
In SACs, the catalytically active single atoms are anchored onto supports such as carbon, metal oxides, or graphene. This configuration allows for precise control over the electronic environment of the active site and a high degree of interaction with reactants. The isolated atoms can facilitate reactions by providing an exact match to the reactants' needs, minimizing the energy barriers for chemical transformations.
Key Features of Single-Atom Catalysts
Single-atom catalysts are characterized by several distinctive features:
- High Catalytic Activity and Selectivity: The unique structure of SACs leads to a high catalytic activity and selectivity, as the active sites are optimally exposed for reaction.
- Efficient Atom Utilization: SACs utilize atoms at the catalytic sites to their maximal potential, which is advantageous for precious metals like platinum or gold, significantly reducing the amount of metal required for the catalyst.
- Controlled Electronic Structure: The electronic properties of SACs can be finely tuned by modifying the support material or the surrounding ligands, enabling the optimization of their catalytic properties for specific reactions.
Advantages of Single-Atom Catalysts
The advantages of SACs are manifold, impacting both the efficiency and sustainability of catalytic processes:
- Resource Efficiency: By maximizing the utilization of active metal atoms, SACs contribute to the more efficient use of scarce and expensive resources.
- Environmental Impact: SACs often operate under milder conditions than traditional catalysts, contributing to reduced energy consumption and lower greenhouse gas emissions.
- Versatility and Wide Application Range: SACs are used in a variety of reactions, including hydrogen production, CO2 reduction, and nitrogen fixation, making them versatile tools in both industrial and environmental settings.
SACs have shown remarkable performance in various chemical reactions, highlighting their versatility and potential for industrial applications. Here are a few specific examples:
- CO2 Reduction: SACs are effective for converting CO2 into valuable chemicals and fuels, offering a path for carbon recycling. For instance, single-atom nickel (Ni) on nitrogen-doped carbon supports has shown high efficiency in reducing CO2 to carbon monoxide (CO), a key intermediate in many chemical syntheses.
- Hydrogen Production: The generation of hydrogen through water splitting is a critical reaction for clean energy. SACs, such as single-atom platinum (Pt) supported on graphene, have demonstrated superior activity and durability for the hydrogen evolution reaction (HER), a half-reaction of water splitting.
- Nitrogen Fixation: Converting nitrogen (N2) from the air into ammonia (NH3) is a fundamental process for producing fertilizers. SACs containing single-atom molybdenum (Mo) catalysts have shown promising activity for nitrogen reduction under ambient conditions, offering a potential energy-efficient alternative to the traditional Haber-Bosch process.
Advantages and Challenges
The high efficiency and selectivity of SACs, coupled with their low metal content, make them attractive for sustainable chemistry. However, challenges remain, including the synthesis of stable SACs under reaction conditions and the need for a deeper understanding of their catalytic mechanisms to further improve their performance.
Applications in Industry and Environmental Processes
SACs have found applications across a broad spectrum of chemical reactions and processes. They are pivotal in energy conversion, pollutant degradation, and the synthesis of fine chemicals, showcasing their potential to drive innovation in sustainable technology and green chemistry.
The exploration of single-atom catalysts is reshaping our understanding of catalytic processes, offering a promising pathway towards more efficient and environmentally friendly chemical synthesis. Their continued development and integration into industrial processes highlight the dynamic intersection of nanotechnology and catalysis, promising significant advancements in material science, energy, and environmental sustainability.
Further Reading
RSC Advances, A minireview on the synthesis of single atom catalysts
Nature Reviews Chemistry, Heterogeneous single-atom catalysis
