Understanding SP Hybridization in Nanotechnology
Definition: SP hybridization is a fundamental concept in chemistry with enormous implications for nanotechnology and materials science. SP hybridization is a concept from molecular orbital theory describing the mixing of one s orbital and one p orbital from the same atom to form two new equivalent orbitals. This process is crucial in understanding the electronic structure and bonding in molecules such as acetylene (C2H2), where carbon atoms exhibit SP hybridization. By grasping SP hybridization, one can better comprehend materials' properties at the nanoscale, which is vital in nanotechnology and materials science.
Understanding SP Hybridization
SP hybridization occurs when one s orbital and one p orbital from the same atom combine to form two new orbitals of equal energy. These new orbitals are linearly oriented 180 degrees apart, leading to the formation of molecules with a linear geometry. In nanotechnology, understanding the SP hybridized structures of carbon, such as in carbon nanotubes and graphene, is essential for exploiting their unique mechanical and electrical properties.
SP hybridization occurs when one s orbital and one p orbital from the same atom combine to form two new orbitals of equal energy. These new orbitals are linearly oriented 180 degrees apart, leading to the formation of molecules with a linear geometry. In nanotechnology, understanding the SP hybridized structures of carbon, such as in carbon nanotubes (which utilize SP hybridized carbon atoms), is essential for exploiting their unique mechanical and electrical properties.
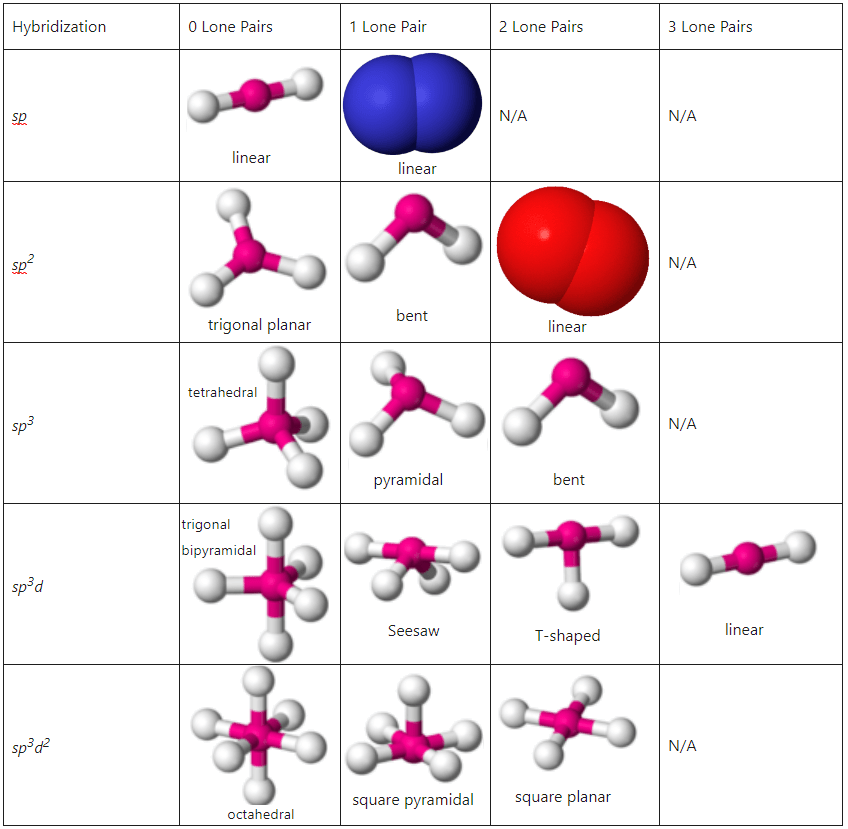
For instance, sp2 means one s orbital combines with two p orbitals, forming three hybrid orbitals with a trigonal planar geometry, while sp3 means one s orbital and three p orbitals combine, creating four hybrid orbitals in a tetrahedral arrangement (see image above).
Characteristics of SP Hybridized Orbitals
Orbitals resulting from SP hybridization are highly directional, allowing for the formation of strong covalent bonds. This directional bonding is critical in creating materials with specific desired properties, such as high strength, conductivity, and resilience at the nanoscale.
Applications in Nanotechnology: A Closer Look at Carbon Nanotubes
One of the most compelling applications of SP hybridization is observed in carbon nanotubes (CNTs). These cylindrical nanostructures are essentially rolled-up sheets of graphene, a single layer of carbon atoms arranged in a hexagonal lattice. The remarkable mechanical strength, electrical conductivity, and thermal stability of carbon nanotubes can be attributed to the SP hybridization of carbon atoms in their structure.
Carbon Nanotubes: A Prime Example
Carbon nanotubes are a prime example of materials benefiting from SP hybridization. In CNTs, each carbon atom is SP hybridized, forming bonds with three other carbon atoms at angles of approximately 120 degrees in the graphene sheet. However, the cylindrical structure of CNTs and the linear combination of orbitals contribute to a unique arrangement where the electrons can delocalize over the entire structure, providing exceptional strength and conductivity. This SP hybridization allows for the formation of strong covalent bonds that are directionally aligned, resulting in a material that exhibits superior tensile strength—many times stronger than steel, yet significantly lighter.
Enhanced Electrical Properties
Moreover, the SP hybridization in carbon nanotubes facilitates the delocalization of electrons, which, in turn, significantly enhances their electrical properties. Depending on their specific structure (single-walled or multi-walled), CNTs can exhibit metallic or semiconducting behavior. This versatility makes them incredibly useful in various applications, from nanoelectronic devices and conductive composites to energy storage and even in the field of nanomedicine for drug delivery systems.
Implications for Material Science and Engineering
The understanding and application of SP hybridization in materials like carbon nanotubes exemplify how fundamental concepts in chemistry and physics can lead to breakthroughs in materials science and engineering. By leveraging the properties conferred by SP hybridized carbon atoms, researchers and engineers can design and fabricate materials and devices with tailored properties for specific applications, pushing the boundaries of what is technologically possible.
The example of carbon nanotubes underscores the significant impact of SP hybridization on the development of advanced materials. It highlights not only the theoretical importance of molecular orbital theory but also its practical implications in creating materials with exceptional properties for a wide range of applications.
Key Benefits in Materials Science
- Enhanced Material Properties: SP hybridization contributes to the development of materials with superior mechanical, thermal, and electrical properties. This is particularly evident in nanoscale materials, where the precise arrangement of atoms is critical for performance.
- Innovative Applications: Understanding SP hybridization facilitates the creation of innovative applications ranging from electronics to biomedicine. For instance, graphene's exceptional electrical conductivity and strength, derived from SP hybridized carbon atoms, have paved the way for its use in flexible electronic displays and biomedical devices.
- Customizable Material Design: The ability to manipulate SP hybridization patterns allows scientists to tailor materials for specific applications, enabling the design of custom nanoscale structures with optimized properties.
Limitations and Challenges
While SP hybridization offers numerous advantages, there are challenges in manipulating and controlling this process at the nanoscale. Precise control over hybridization patterns is required to achieve desired material properties, posing a significant challenge in nanomaterial synthesis.
Recent Advances in Understanding SP Hybridization
Recent research has focused on better understanding and controlling SP hybridization in various materials. Advances in computational chemistry and materials science have enabled more accurate predictions and manipulations of SP hybridized structures, leading to the development of new materials with unprecedented properties.
The exploration of SP hybridization continues to be a dynamic area of research in nanotechnology, with ongoing studies aimed at uncovering new ways to leverage this phenomenon for the development of advanced materials and devices.
Further Reading
Energy Storage Materials, Carbon nanomaterials with sp2 or/and sp hybridization in energy conversion and storage applications: A review
Journal of Chemical reviews, A Critical History of Hybrid Atomic Orbitals and Hybridization
