| Posted: May 06, 2015 |
One step closer to nano-sized cancer drug delivery
|
|
(Nanowerk News) When you take a drug, it travels through your bloodstream, dissolving and dispersing, and eventually reaching its designated target area.
|
|
But because the blood containing the drug travels all round your body only a small percentage of the initial dose actually reaches the desired location.
|
|
For over-the-counter drugs like paracetamol or ibuprofen, with very few side-effects, this doesn’t matter too much.
|
|
But when it comes to cancer drugs, which can affect healthy cells just as much as cancer cells, this process can cause big problems.
|
|
Partly because drugs are diluted in their blood, cancer patients need to take these drugs in particularly high doses – and this can cause seriously unpleasant side effects.
|
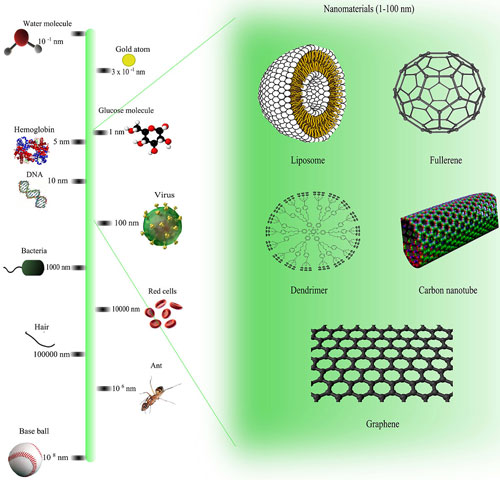 |
| Comparison of nanomaterials sizes. (click on image to enlarge)
|
|
But Professor Sonia Trigueros, co-director of the Oxford Martin Programme on Nanotechnology, is inching closer to developing a nano-scale drug delivery system with the aim of specifically targeting cancer cells.
|
|
Working with a team of chemists, engineers and physicists, Trigueros has embarked on an ambitious mission to tackle cancer at the ‘nano’ level – less than 100 nanometers wide.
|
|
There’s still a long way to go, but Trigueros is making decent headway, and has recently tackled a major problem of working at a nano level. And at this year’s Wired Health conference – which looked at the future of health care, wellbeing and genomics – she told us about her recent progress, and her visions for the future.
|
|
At the nano level
|
|
Some of us will remember the periodic table displayed in our science classrooms which told us about the properties of each element. But working on a nano level everything changes, and elements behave completely differently.
|
|
Elements have different properties at the nano level than they do at the micro level, explained Prof Trigueros to the Wired Health 2015 audience.
|
|
This poses big problems for researchers trying to make nano-scale devices, which can be made out of a number of different materials, including gold, silver and carbon. All these materials are highly unstable at the nano level.
|
|
“After you make the nanostructures you only have minutes to a couple of days to work,” she said. They are really unstable, especially when you put them in water.”
|
|
This isn’t ideal, considering our bodies are made up mostly of water.
|
|
Trigueros’ recent work has focused on trying to stabilise tiny tubes made of carbon, called carbon nanotubes, which hold drugs inside the tube so they can be delivered into cancer cells.
|
|
She has now found a way of keeping them stable for more than two years and in temperatures up to 42ºC.
|
|
To do this, she wraps DNA around the structures, like a tortilla wraps around the fillings of a burrito.
|
|
While this accomplishes the goal of keeping the nanostructures stable inside the body this doesn’t do much good if the DNA can’t unwrap to deliver the drugs. But, according to Trigueros, she has shown that, once inside a cell, the DNA easily unwinds and releases its payload.
|
|
Truly targeted drug delivery
|
|
So how does it all work? How do the drugs get into the cancer cells? Trigueros’s nanotubes exploit the differences between cancer cells and healthy cells – in this case, differences in the membranes that hold them together.
|
|
“Cancer cells are more permeable than normal cells so the nanotubes can get through the cell membrane. And once they are in, they unwrap and deliver drug,” explained Trigueros.
|
|
Exploiting differences in their permeability is one way to target the cancer cells, but Trigueros explains that there is more than one way to create a truly targeted drug delivery system.
|
|
“We can attach whatever we want on DNA,” she said. “So you can attach a protein that recognises cancer cells”.
|
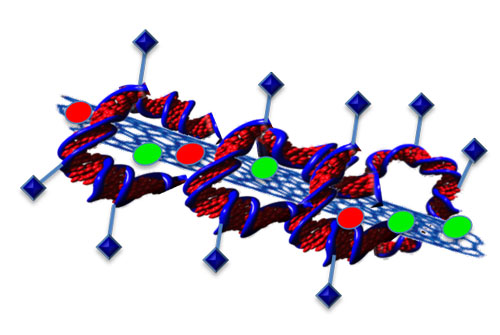 |
| Attaching proteins to DNA could create a truly targeted drug delivery system.
|
|
From theory to reality
|
|
While this all sounds great in theory, will it actually work in reality?
|
|
Trigueros has now started preliminary tests on laboratory grown lung cancer cells, she told us during an interview. And this has shown tentative promise, she says, citing unpublished data on their effectiveness at killing these cells in the lab.
|
|
Others are cautiously optimistic. “This is a really exciting prospect,” says Professor Duncan Graham, nanotechnology expert and advisor to Cancer Research UK.
|
|
“A common concern with carbon nanotubes is toxicity, but when coated with DNA this concern could be removed,” he explains, “and it also addresses a fundamental issue, which is that they collect into clusters that become a solid mass and so are unable to leave the body.”
|
|
In theory, once Trigueros’s nanotubes have finished their job they are tiny enough (50 nanometres) to be excreted through urine.
|
|
This isn’t the first time carbon nanotubes have been used in cancer research: a US research team has used them, for example, to target and collect images of tumours in mice. But the combination of drug delivery and cancer-specific targeting is what interests Professor Graham.
|
|
“Unlike previous work using carbon nanotubes, this approach is set to target the tumour specifically, potentially meaning fewer side effects and a lower dosage. I look forward to seeing this in animal models which is where the real proof of activity lies,” he said.
|
|
But he’s cautious, stressing that Trigueros’s work has not yet been peer-reviewed and published.
|
|
Next steps
|
|
Next Trigueros is aiming towards starting animal trials and, eventually, she wants to begin clinical trials in patients – that is if everything goes well.
|
|
She hopes to focus on how nanostructures could be used to cross the blood-brain barrier – the brain’s highly selective ‘bouncer’ that only lets certain molecules across. This has been notoriously difficult to get past, making targeting cancers in the brain more difficult.
|
|
But there is a still a long way to go and a lot of problems to tackle. In the shorter term, we’ll be keeping an eager eye on her drug delivery research, as her ideas continue to develop.
|

