| Posted: Jun 04, 2015 |
Water near a water-repelling surface cannot dissolve salts
|
|
(Nanowerk News) Researchers at the University of Tokyo have demonstrated that water near a water-repelling surface has significantly less capability to dissolve salts (Science, "Subnanoscale hydrophobic modulation of salt bridges in aqueous media").
|
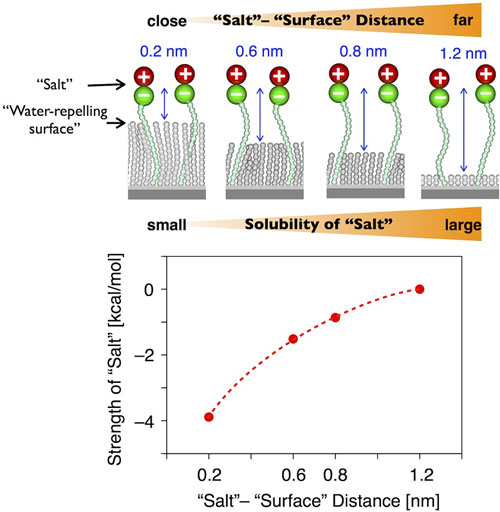 |
| Salt and a water-repelling surface. Water near a water-repelling surface is less likely to dissolve salts. (Image: Yoshimitsu Itoh, Takuzo Aida)
|
|
Surface pollution is a problem that is found everywhere, such as on dirty clothes in everyday life and shellfish on a ship’s bottom. Since the initial step of pollution starts from adhesion of a pollutant onto a surface, designing antipollution surfaces require an in-depth understanding of surface adhesion at the nanoscale. However, scientific understanding of surface adhesion is still very limited.
|
|
The research group of Dr. Yoshimitsu Itoh and Dr. Takuzo Aida at the University of Tokyo, Graduate School of Engineering, Department of Chemistry and Biotechnology discovered that water near a water-repelling surface has significantly less capability to dissolve salts. They utilized a plate where they could place a salt at a defined distance from a water-repelling surface and measured the solubility of salt in water. They found is that when the distance from the water-repelling surface decreases, the salt becomes less soluble. This phenomenon is pronounced at distances of less than one nanometer. One nanometer is the distance at which a substance sticks to a surface.
|
|
“Water near a water-repelling surface is less able to dissolve salts.” This unique understanding will force a revision of the common sense regarding salt dissolution. This will pave the way not only for designing novel antipollution surfaces, but also for designing drugs, which also requires a precise understanding of interactions between water-attracting and water-repelling parts of molecules.
|

