| Posted: Oct 18, 2018 | |
Placing atoms for optimum catalysts(Nanowerk News) Fuels, plastics, and other products are made using catalysts, materials that drive chemical reactions. To design a better catalyst, scientists must get the right atoms in the right spot. |
|
| Positioning the atoms can be difficult, but new research makes it easier. Researchers determined the exact location of single oxygen atoms, which act like anchors for catalysts. In the case of a layer of carbon atoms atop a metal support, single oxygen atoms appear in predictable spots. | |
| Knowing where the atomic anchors are, the team can create patterns of catalytic atoms, designing what’s needed to get the job done. | |
| Creating catalysts that make reactions faster and less wasteful means designing the catalysts from the bottom up. Rather than search among countless possibilities, scientists want to design the right structures at a molecular level. | |
| New fundamental research (Journal of the American Chemical Society, "Formation of supported graphene oxide: Evidence for enolate species") shows scientists how to take advantage of precise spots—where the oxygen atoms bind on graphene—to build model catalysts. | |
| This research redefines what is known about oxygen binding, which is vital to creating hard-working catalysts. | |
 |
|
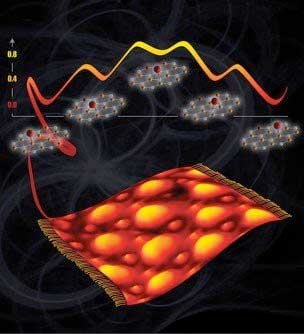
| On top of a graphene sheet thrown like a carpet over a ruthenium metal surface, oxygen atoms (red spheres in top image, small bumps on bottom image) change their binding preference to only one carbon at a time instead of two. (Image: Vassiliki-Alexandra Glezakou and Cortland Johnson, Pacific Northwest National Laboratory) | |
| The team began with a flat piece of ruthenium metal. On top of the metal, they grew graphene, which is a one-atom-thick layer of carbon. In this structure, some carbon atoms bind to the metal, while others don't. By combining experimental and computational resources, the team examined these carbon atoms. | |
| They showed that single oxygen atoms, which act as ideal spots to attach catalytic sites, bind preferentially to carbon atoms that are close to the underlying metal but not bound to it. Less preferred sites for oxygen binding are between two carbon atoms; carbon atoms that are, in turn, bound to ruthenium; and untethered carbon atoms far from the ruthenium. | |
| This research redefines what scientists know about oxygen binding to carbon atoms on metal-supported graphene. The work is vital to designing efficient, selective catalysts. |
| Source: U.S. Department of Energy, Office of Science | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
