| Dec 10, 2018 | |
Proteins imaged in graphene liquid cell have higher radiation tolerance(Nanowerk News) Electron microscopy is one of the main methods used to examine protein structure. Studying these structures is of key importance to elucidate their function feeding fundamental information into a number of fields such as structural biology, cell biology, cancer research, and other biomedical fields. It also enhances the understanding of biomineralization. |
|
| A new option for imaging proteins is liquid-phase electron microscopy (LPEM), which is capable of imaging native (unstained) protein structure and other samples such as nanomaterials or cells in liquid. This technology was developed over the past fifteen years. | |
| Until recently, it debated whether the radiation tolerance of liquid samples would be better or worse compared to amorphous ice. | |
| In their recent publication in Nano Letters ("Reduced radiation damage in transmission electron microscopy of proteins in graphene liquid cells"), Sercan Keskin and Niels de Jonge from the INM-Leibniz Institute for New Materials now demonstrate, that the radiation tolerance is increased by an order of magnitude compared to a sample in ice. | |
 |
|
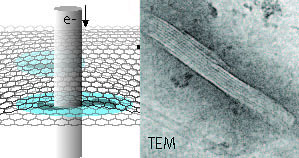
| Schematic representation of hydrated microtubule proteins encapsulated between two graphene layers imaged by transmission electron microscopy (TEM). An example of a TEM image of a microtubule is shown at the right The interior lining reflects the protofilament structure of the polymeric microtubule. Free within this press release. (Image: INM, Niels de Jonge) | |
| This result was achieved by preparing a microtubule sample in a graphene liquid cell. Essential was to use a low as possible rate at which the electron beam irradiation was applied. | |
| Traditionally, samples were fixed, stained with a metal to enhance their contrast, subsequently dried, embedded in plastic, cut in thin sections, and then imaged in the vacuum environment required for electron microscopy. | |
| Cryo electron microscopy overcomes the drawbacks associated with this sample preparation and provides the means to study proteins in a close to native hydrated state by preparing them in amorphous ice. However, a key imitating is the high sensitivity of the samples to electron beam irradiation, so that statistical noise in the image prevents high resolution and many ten thousand noisy images of identical structures need to be imaged in order to resolve the structure. |
| Source: INM - Leibniz-Institut für Neue Materialien | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
