| Feb 06, 2019 | |
The subtle, but significant, role of surfaces in ion stickiness(Nanowerk News) How ions stick to surfaces greatly influences vital processes in everything from water purifiers to batteries. For decades, scientists have debated the mechanics of such binding, or adsorption. Certain ions in water didn’t adsorb to a surface as predicted. |
|
| Now, scientists know why. By studying a graphene surface immersed in water, scientists showed that an interaction between the graphene and an ion drives the adsorption, unlike the case for the air/water interface (PNAS, "Mechanism of ion adsorption to aqueous interfaces: Graphene/water vs. air/water"). | |
 |
|
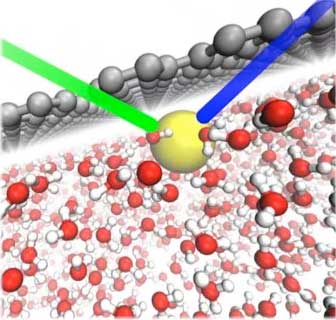
| In water (red and white structures), the direct interaction between graphene (gray) and a thiocyanate (SCN–) ion (yellow) causes the ion to adsorb to the surface. The green and blue lines represent the incident and reflected light pulses during ultraviolet second harmonic generation spectroscopy. (Image: Richard Saykally and Phillip Geissler, University of California, Berkeley and Lawrence Berkeley National Laboratory) | |
| When it comes to ion adsorption, there are subtle, but important, differences at the molecular scale. Thus, scientists need to consider these differences when working with ions and surfaces in water. These interactions are present in everything from battery construction to nerve signaling to cloud formation. | |
| In many areas of science and technology, the adsorption of ions to aqueous interfaces plays a key role. For a long time, chemistry and physics texts have stated that all ions are repelled from interfaces between water and a hydrophobic material. | |
| However, new experiments and computer simulations have shown that certain ions, known as chaotropic ions, don’t follow that pattern. Researchers investigated why those ions adsorb differently. They examined thiocyanate ions sticking to a sheet of graphene (a thin, noncharged carbon surface) immersed in water. | |
| Using deep ultraviolet second harmonic generation measurements of the ion, along with associated computer simulations, the team determined the free energy of ion adsorption. While the free energy involved in adsorbing the ion to the graphene/water interface is nearly identical to the corresponding value for an air/water interface, the research shows the mechanisms involved to be totally different. | |
| While adsorption at the air/water interface is dominated by solvent repartitioning, in which the water molecules change position and orientation near the ion, direct interactions of the ion with graphene dominate in the case of graphene/water interfaces. |
| Source: U.S. Department of Energy, Office of Science | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
