| Mar 01, 2019 | |
Nanoparticles influence their liquid environment(Nanowerk News) These days, nanoparticles finely distributed in suspensions are used in many areas – for example in cosmetic products, in industrial catalysts, or in contrast agents for medicinal examinations. |
|
| For the first time, a research team from the University of Bayreuth has managed to precisely determine the interrelationships of magnetic nanoparticles with the liquid surrounding them, even down to the atomic level. As it turns out, it is mainly a question of the crystalline structure of the nano-particle as to how water molecules in their immediate vicinity re-align themselves. | |
| The scientists have presented their findings in the journal Nature Communications ("Atomic insight into hydration shells around facetted nanoparticles"). | |
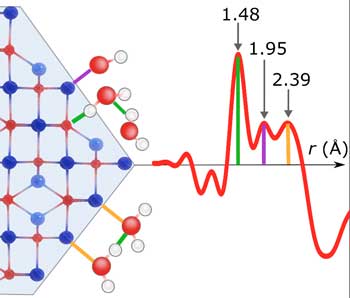 |
|
| Schematic representation of the adsorption of water molecules on the crystal surfaces of magnetic iron oxide nano-particles. The red curve shows the measuring signal of the pair distribution function. (© Nature Communications) | |
| On the basis of theoretical and experimental studies, the research community had long assumed that the molecules of a liquid group themselves around a solid nanoparticle like a shell. Within these so-called solvation shells – in the case of water solutions they are also referred to as hydration shells – three to five layers can be distinguished, corresponding to the arrangement of the liquid molecules. Yet up to now, only information about number and size of these layers was accessible. | |
| Consequently, the team of scientists working with Bayreuth’s junior professor Mirijam Zobel, took a closer look at the atomic and molecular structures of these layers in a series of experiments. | |
| To this end, high-energy X-ray measurements were carried out using the Diamond Lightsource, an electron synchrotron in Great Britain. The investigations concentrated on magnetic nanoparticles, widely used these days in biomedicine, especially in targeted drug release, and in magnetic resonance imaging. | |
| In doing so, the researchers discovered that even the distances separating the atoms of the water molecules that surround a nanoparticle can be precisely measured. In this way, it finally became apparent how water molecules adhere to the nanoparticle: in some cases by means of dissociative bonds, in other cases via molecular adsorption. | |
| “It was surprising for us that water in the vicinity of tiny magnetic iron oxide nanoparticles arranged itself just like on level iron oxide surfaces on the macroscopic level. We were able to prove that the way in which liquid molecules arrange themselves in the vicinity of a nanoparticle depends primarily on the crystalline structure of the nanoparticle. In contrast, the small organic molecules found on the surfaces of nanoparticles don’t have a direct influence on the arrangement of the liquid molecules”, project leader Mirijam Zobel explains. | |
| “These are important insights for further research and its applications. Because these organic molecules, with which the nanoparticles are stabilized, serve as anchor points when, in biomedical applications, the nanoparticles are loaded, with anti-bodies, for example. Hence for the release of such medicinal agents, it is of crucial significance to understand in detail the influence of these molecules on the characteristics and behaviour of the nanoparticles”, Bayreuth PhD student Sabrina Thomä M.Sc. explains, lead author of the study published in Nature Communications. | |
| Junior professor Mirijam Zobel continues: “The study of solvation shells around nanoparticles has meanwhile established itself as a subject in its own right all around the world. We’re convinced that the method we have developed, which we deployed in the new study, can be used more generally. Indeed, in future we will be able to achieve many more exciting insights into “Solvation Science”, for example in the areas of catalysts and nucleation.” | |
Background |
|
| To determine the structures of the liquid molecules in solvation shells, the research team centred around Prof. Dr. Mirijam Zobel made use of an X-ray based research method referred to as Pair Distribution Function (PDF). A high-performance X-ray diffractometer, which is set to advance the use of this method so important to the nanosciences, was recently installed on the Campus of the University of Bayreuth. |
| Source: University of Bayreuth | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
