Nanoparticles Types, Properties and Uses
Table of Contents
- Nanoparticle Definition
- Nanoscale Dimensions
- Materials Behave Differently At The Nanoscale
- Different Types Of Nanoparticles
- Synthesis of Nanoparticles
- Characteristics of Nanoparticles
- Applications of Nanoparticles
Synthetic nanoparticles are artificially manufactured particles that have dimensions in the nanoscale range (typically 1 to 100 nanometers). These particles can be made of a wide variety of materials including metals, metal oxides, polymers, and dendrimers. Synthetic nanoparticles are used in a range of applications, such as in electronics, energy, medicine, and catalysis, due to their unique properties that arise from their small size and high surface area to volume ratio. Synthetic nanoparticles can be manufactured using a variety of methods, including chemical synthesis, physical vapor deposition, and material synthesis by template-assisted methods.
Nanoparticles are now being used for instance in the manufacture of scratchproof eyeglasses, crack-resistant paints, anti-graffiti coatings for walls, transparent sunscreens, stain-repellent fabrics, self-cleaning windows and ceramic coatings for solar cells. Nanoparticles can contribute to stronger, lighter, cleaner and 'smarter' surfaces and systems.
Synthetically produced nanoparticles play an important role in nanotechnology and find use in many applications. This includes dispersions in gases (e.g. as aerosols), as ultrafine powder, for thin films, distributed in fluids (dispersed, for example ferrofluids) or embedded in a solid body (nanocomposites).
One striking example of what these novel coatings can do is LiquiGlide, which is about to end consumers' infamous battle with the ketchup bottle:
There are numerous ways of nanoparticle classifications: Natural or man-made? Synthetically produced or by-products? Organic or inorganic? By size; shape; surface properties; or functionalization.
The diversity of synthetic nanoparticles is considerable. They are distinct in their properties and applications. In addition to their size, synthetic nanoparticles vary in chemical composition, shape, surface characteristics and mode of production.
In addition to commercially produced nanomaterials (check out our vast library of nanomaterials here), many nanoparticles are not solely a product of modern technology, but are also created by natural processes such as volcano eruptions or forest fires. Naturally occurring nanoparticles also include ultrafine sand grains of mineral origin (e.g. oxides, carbonates). Large quantities of nanoparticles are unintentionally created by the combustion of diesel fuel (ultrafine particles) or during barbecuing.
Nanoparticle Definition
The term nanoparticle is a mixture of the words ìnanosî (Greek: dwarf) and ìparticulumî (Latin: particle). In the scientific context, ìnanoî primarily refers to a specific order of magnitude, namely 10-9 in the metric system. This can refer to a volume, a weight or a unit of time, whereby a nanometer (nm = 10-9 meters) corresponds to one billionth of a meter. To illustrate this more graphically, a nanometer has the same relation to a meter as the diameter of a tennis ball to the diameter of the Earth. Check out the Nanowerk metric prefic table and "scale of things".
You will also come across the term nanocluster quite often. These are small agglomerates of atoms and molecules, consisting of a few to some thousands of units and have diameters mostly in the single nanometer scale. The name nanoparticle is often used when speaking of bigger clusters with diameters from several nanometers to several hundreds of nanometers, but the distinction between a cluster and a nanoparticle is not well-defined.
Nanoscale Dimensions
Scientists classify nanomaterials based on the number of dimensions of a material, which are outside the nanoscale (<100 nm) range.
Accordingly, in zero-dimensional (0D) nanomaterials all the dimensions are measured within the nanoscale (no dimensions are larger than 100 nm). Most commonly, 0D nanomaterials are nanoparticles.
In one-dimensional nanomaterials (1D), one dimension is outside the nanoscale. This class includes nanotubes, nanorods, and nanowires.
In two-dimensional nanomaterials (2D), two dimensions are outside the nanoscale. This class exhibits plate-like shapes and includes graphene, nanofilms, nanolayers, and nanocoatings.
Three-dimensional nanomaterials (3D) are materials that are not confined to the nanoscale in any dimension. This class can contain bulk powders, dispersions of nanoparticles, bundles of nanowires, and nanotubes as well as multi-nanolayers.
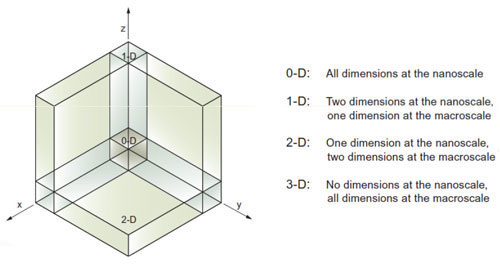
Classification of nanoscale dimensions. (Source: Tallinn University of Technology)
Materials Behave Differently At The Nanoscale
Two principal factors cause the properties of materials in nanoparticle size to differ significantly from their bulk form: increased relative surface area, and quantum size effects. These factors can change or enhance properties such as reactivity, strength and electrical characteristics.
Surface Area
As a particle decreases in size, a greater proportion of atoms are found at the surface compared to those inside. At a particle diameter of 10 nm, 20 % of the approximately 30 000 atoms of the entire particle are positioned on its surface; at a particle diameter of 5 nm, the value increases to 40 %, and at 1 nm diameter, almost all of the atoms are on the surface. The surface atoms, as opposed to those inside the material, have fewer direct neighbors and therefore contain so-called unsaturated bonds. These are responsible for the higher reactivity of the particle surface.
Thus nanoparticles have a much greater surface area per unit mass compared with larger particles. As growth and catalytic chemical reactions occur at surfaces, this means that a given mass of material in nanoparticulate form will be much more reactive than the same mass of material made up of larger particles.

To understand the effect of particle size on surface area, consider an American Silver Eagle coin. This silver dollar contains 31 grams of coin silver and has a total surface area of approximately 3000 square millimeters. If the same amount of coin silver were divided into tiny particles – say 10 nanometer in diameter – the total surface area of those particles would be 7000 square meters (which is equal to the size of a soccer field – or larger than the floor space of the White House, which is 5100 square meters). In other words: when the amount of coin silver contained in a silver dollar is rendered into 10 nm particles, the surface area of those particles is over 2 million times greater than the surface area of the silver dollar!
Increased reactivity is the basis for numerous applications. Precisely controlling particle diameter yields a new generation of catalysts with high selectivity; such catalysts accelerate chemical processes.
This high reactivity also reduces the melting point, so that using nanoparticular raw materials would reduce the firing temperature in the case of ceramics. More importantly, the composites (solids composed of various materials) would shrink less during the hardening process, a particularly important feature in dental prosthetics for example.
Quantum Size Effects
The so-called quantum size effect describes the physics of electron properties in solids with great reductions in particle size. This effect does not come into play by going from macro to micro dimensions. However, it becomes dominant when the nanometer size range is reached.
Quantum effects can begin to dominate the behavior of matter at the nanoscale – particularly at the lower end (single digit and low tens of nanometers) – affecting the optical, electrical and magnetic behavior of materials.
The causes of these drastic changes stem from the weird world of quantum physics. The bulk properties of any material are merely the average of all the quantum forces affecting all the atoms that make up the material. As you make things smaller and smaller, you eventually reach a point where the averaging no longer works and you have to deal with the specific behavior of individual atoms or molecules – behavior that can be very different to when these atoms are aggregated into a bulk material.
Materials reduced to the nanoscale can suddenly show very different properties compared to what they show on a macroscale. For instance, opaque substances become transparent (copper); inert materials become catalysts (platinum); stable materials turn combustible (aluminum); solids turn into liquids at room temperature (gold); insulators become conductors (silicon).
Varying nanoparticle size not only modifies reactivity but can also alter the optical characteristics such as transparency, absorption, luminescence and scattering. Although particles measuring only a few nanometers in diameter lie far below the wavelength range of visible light (380 to 780 nm), they can absorb light of specific wavelengths.
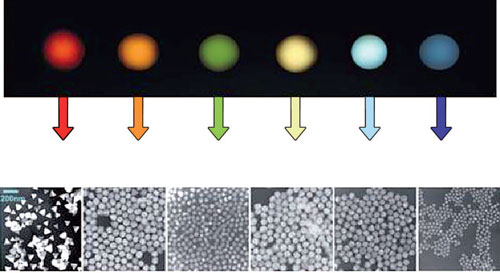
These effects can only be understood on a quantum mechanics level. In the case of quantum dots, which are composed of semiconductor materials, particle size can be used to adjust the wavelength of the fluorescence, for example. These optical features make nanoparticles especially interesting for applications in optoelectronics, cosmetics and medical diagnostics.
Different Types Of Nanoparticles
Nanoparticles can be classified into different types according to the size, morphology, physical and chemical properties. Some of them are carbon-based nanoparticles, ceramic nanoparticles, metal nanoparticles, semiconductor nanoparticles, polymeric nanoparticles and lipid-based nanoparticles.
Nanoparticles types are commonly divided in two main groups: organic and inorganic. The first group includes micelles, dendrimers, liposomes, hybrid and compact polymeric nanoparticles. The second group includes fullerenes, quantum dots, silica and metal nanoparticles.
Another way of classifying nanoparticles is based on their morphology, size and chemical properties. Based on physical and chemical characteristics, some of the important classes of nanoparticles are:
Carbon-based – (fullerenes, carbon nanotubes, graphene, carbon dots). These materials are of great interest due to their electrical conductivity, high strength, structure, electron affinity, and versatility.
Metal – They are purely made of the metals precursors. Due to localized surface plasmon resonance (LSPR) characteristics, these these possess unique optoelectrical properties.
Ceramic – These inorganic nonmetallic solids are getting great attention of researchers due to their use in applications such as catalysis, photocatalysis, photodegradation of dyes, and imaging applications.
Semiconductor – Semiconductor materials possess properties between metals and non-metals and have wide bandgaps. Bandgap tuning results in significant alteration in their properties. Therefore, they are very important materials in photocatalysis, photo optics and electronic devices.
Polymers – Scientists have developed many techniques to synthesize polymeric nanoparticles for a wide range of applications including surface coating, sensor technology, catalysis, and nanomedicine.
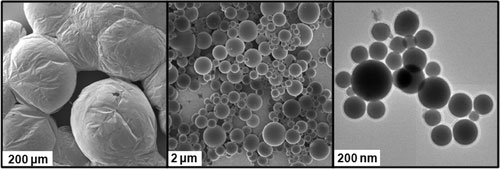
Liquid-phase eutectic gallium–indium (EGaIn) alloy nanoparticles.
Lipids – These NPs contain lipid moieties and are used in many biomedical applications as drug carriers (the mRNA Covid-19 vaccines are using lipid nanotechnology). Lipid nanoparticles are also regarded as highly promising systems for delivering nucleic acids in gene therapy.
Synthesis of Nanoparticles
Specific synthesis processes are employed to produce the various nanoparticles, coatings, dispersions or composites. Defined production and reaction conditions are crucial in obtaining such size-dependent particle features. Particle size, chemical composition, crystallinity and shape can be controlled by temperature, pH-value, concentration, chemical composition, surface modifications and process control.
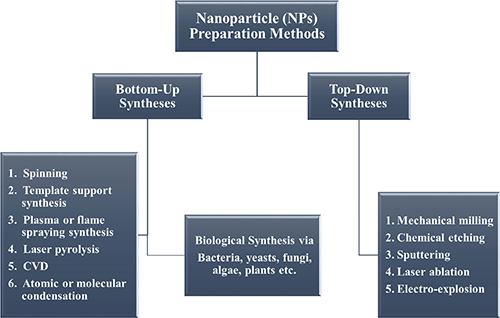
Two basic strategies are used to produce nanoparticles: top-down and bottom-up. Read all about it in our detailed article on how nanoparticles are made.
In general, the term top-down refers here to the mechanical crushing of source material using a milling process. In the bottom-up strategy, structures are built up by chemical and self-assembly processes. The selection of the respective process depends on the chemical composition and the desired features specified for the nanoparticles.
Characteristics of Nanoparticles
Researchers are still challenged by the task of determining the physicochemical properties of nanoparticles and exploring their structure-function relationships. A key limitation is their ability to fully investigate the nanoscale realm: Different characterization techniques are based on different physical properties, therefore only providing a partial picture of the nanoparticle characteristics. Making matters more challenging yet, the characterization methods themselves can directly affect the measured quantities of nanoparticles.
Nanoparticles exist in various chemical compositions ranging from micelles to metal(oxide)s, from synthetic polymers to large biomolecules. Each of these materials features a completely different chemistry, which can be analyzed by a variety of methods including optical spectroscopy, X-ray fluorescence and absorbance, Raman spectroscopy, and solid-state NMR.
However, often the behavior of nanoparticles is largely governed by their nanometer dimensions. As such, throughout nanoparticle characterization, the investigation of size, shape, surface charge and porosity is a fundamental step for fully understanding and predicting their behavior.
Various analytical techniques are employed to characterize the key properties of nanoparticles. >Electron microscopy methods such as >Transmission Electron Microscopy (TEM) and >Scanning Electron Microscopy (SEM) provide detailed images that allow direct visualization of nanoparticle size, morphology, and dispersion state. >Dynamic Light Scattering (DLS) is widely used to determine the hydrodynamic size distribution of nanoparticles in suspension. >X-ray diffraction (XRD) provides information on the crystalline structure and grain size. Brunauer-Emmett-Teller (BET) analysis is used to measure the specific surface area of nanoparticles based on gas adsorption. Zeta potential measurements shed light on the surface charge and stability of nanoparticles in solution. >Fourier-Transform Infrared Spectroscopy (FTIR) and >X-ray Photoelectron Spectroscopy (XPS) provide information about the chemical composition and surface functionalization of nanoparticles. Employing a combination of these complementary techniques enables researchers to gain a comprehensive understanding of the physicochemical properties of nanoparticles.
Check out our article on how to characterize nanoparticles.
Applications of nanoparticles
The fields of application for nanoparticles are wide ranging (also check out our primer How does nanotechnology work). They play a major role in materials development. The great expectations we place on today's nanoparticle-containing materials is based on the hope that the different material properties such as conductivity, weight, stability, flexibility, heat resistance etc. can be specified independently from one another.
Nanoparticle applications also have been introduced on the market in paints, polymer nanocomposites and nanopigments. Numerous nanotechnology products have been on the market for some time now. In the chemical sector this includes Carbon Black (soot particles), for printing black; in the automobile sector this includes scratch-resistant paints, filler in tires and anti-reflective layers. Nanoparticles exist for highly efficient hydrogen storage systems, self-healing materials, and coatings that switch their color using sensor technology.
Nanoparticles are increasingly found in a wide range of commercial products across various sectors. In the automotive industry, nanoparticles are used in catalytic converters to reduce exhaust emissions, as well as in high-performance tires to improve traction and durability. The electronics sector employs nanoparticles in semiconductor fabrication, magnetic recording media, and touchscreens. In the textile industry, nanoparticles are incorporated into fabrics to impart antimicrobial properties, UV protection, and stain resistance. The construction industry uses nanoparticles in advanced insulation materials, self-cleaning windows, and high-strength concrete. In the cosmetics and personal care sector, nanoparticles are found in sunscreens, moisturizers, and hair care products. The food industry utilizes nanoparticles to enhance nutrient delivery, improve packaging, and extend shelf life. These examples highlight the growing prevalence of nanoparticles in everyday consumer products, driven by their unique properties and the ability to engineer materials at the nanoscale.
In the life sciences, nanoparticles are used for biochips as well as for so-called markers. They are also used in sunscreens and cosmetic products. In medical diagnostics, nanoparticles are increasingly being used as contrast media; they are also a tool in cancer therapy as drug delivery agents. Nanoparticles are promising in regenerative medicine, for example in tissue cultures.
