Carbon Dots: Luminescent Nanomaterials for Diverse Applications
What are Carbon Dots?
Carbon dots (CDs) are a class of fluorescent carbon nanomaterials with sizes below 10 nm. They are composed of sp2/sp3 hybridized carbon cores with abundant surface functional groups. CDs exhibit unique properties such as excellent photoluminescence, high photostability, low toxicity, and good biocompatibility, making them promising candidates for various applications in bioimaging, biosensing, photocatalysis, and optoelectronics.
Carbon dots can be broadly categorized into four types based on their structures and synthesis methods:
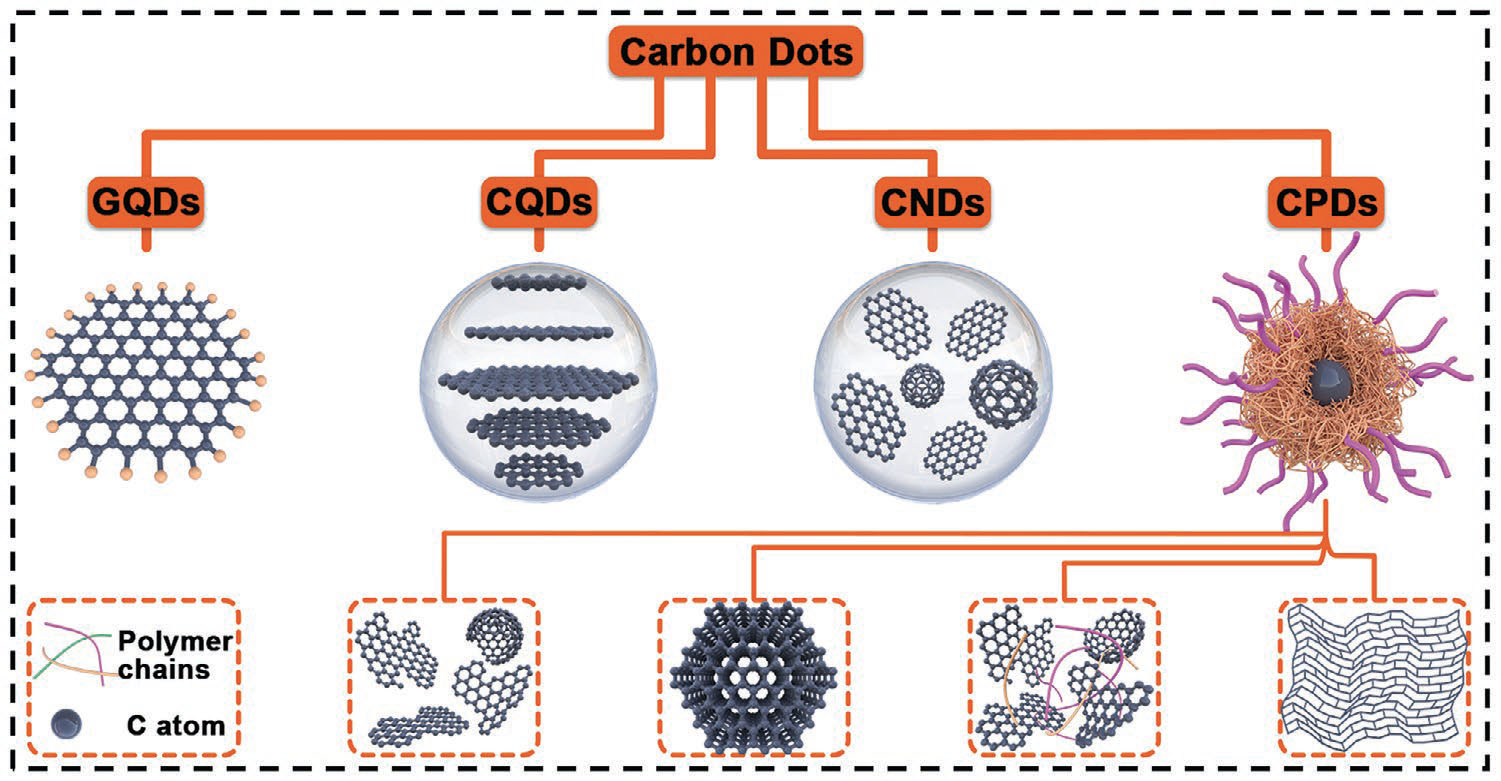
- Graphene Quantum Dots (GQDs): GQDs are derived from graphene or graphene oxide sheets and have a well-defined graphene lattice structure. They typically exhibit strong quantum confinement effects and possess excellent optical properties.
- Carbon Quantum Dots (CQDs): CQDs are spherical nanoparticles with a partially crystalline carbon core and abundant surface functional groups. They are often synthesized via bottom-up approaches using small organic molecules as precursors.
- Carbon Nanodots (CNDs): CNDs are amorphous or partially crystalline quasi-spherical nanoparticles with a disordered carbon core. They are commonly prepared by top-down methods such as electrochemical synthesis or laser ablation.
- Carbonized Polymer Dots (CPDs): CPDs are prepared by the carbonization of polymer precursors, resulting in a polymer/carbon hybrid structure. They inherit the properties of both the polymer and carbon components, offering additional functionality and versatility.
Synthesis Methods
Several methods have been developed for the synthesis of carbon dots, which can be broadly classified into two categories:
- Top-Down Approaches: These methods involve the breakdown of larger carbon structures, such as graphite, carbon nanotubes, or biomass, into smaller carbon dots. Techniques such as arc discharge, laser ablation, electrochemical oxidation, and chemical oxidation are employed in top-down synthesis.
- Bottom-Up Approaches: Bottom-up methods involve the synthesis of carbon dots from molecular precursors through processes like hydrothermal/solvothermal treatment, microwave-assisted synthesis, and pyrolysis. These methods allow for better control over the size, composition, and surface functionality of the resulting carbon dots.
Optical Properties
Carbon dots exhibit remarkable optical properties, including:
- Photoluminescence: CDs show intense and tunable photoluminescence, with emission colors ranging from blue to red depending on their size, composition, and surface states. The photoluminescence of CDs is attributed to various mechanisms, such as quantum confinement, surface defects, and molecular fluorophores.
- Excitation-Dependent Emission: Many carbon dots display excitation-dependent emission, where the emission wavelength shifts with changes in the excitation wavelength. This property enables the use of CDs as multi-color imaging agents and ratiometric sensors.
- High Photostability: Compared to traditional organic dyes and quantum dots, carbon dots exhibit excellent photostability, resisting photobleaching and blinking under prolonged irradiation. This stability makes them suitable for long-term imaging and sensing applications.
Applications of Carbon Dots
Carbon dots have found diverse applications in various fields, exploiting their unique optical and physicochemical properties:
Bioimaging and Biosensing
The excellent photoluminescence, biocompatibility, and low toxicity of carbon dots make them promising fluorescent probes for bioimaging and biosensing. CDs have been used for in vitro and in vivo imaging of cells and tissues, as well as for the detection of various analytes such as metal ions, small molecules, and biomolecules (e.g., proteins, DNA).
Photocatalysis
Carbon dots have shown potential as photocatalysts for various chemical reactions, including the degradation of organic pollutants, water splitting for hydrogen production, and CO2 reduction. The photocatalytic activity of CDs arises from their ability to generate reactive oxygen species upon light irradiation and their efficient charge separation and transfer properties.
Optoelectronics
The unique optical and electronic properties of carbon dots make them attractive materials for optoelectronic applications. CDs have been explored as phosphors for light-emitting diodes (LEDs), sensitizers for solar cells, and active layers in photodetectors and photovoltaic devices.
Challenges and Future Perspectives
Despite the significant progress in carbon dot research, several challenges remain to be addressed. One of the main challenges is the limited understanding of the precise structure-property relationships of CDs, which hinders the rational design of CDs with desired properties. Additionally, the development of large-scale and reproducible synthesis methods is crucial for the commercialization of CD-based products.
Future research on carbon dots will focus on the development of multi-functional CDs with enhanced optical properties and targeted functionalities. The integration of CDs with other nanomaterials, such as metal nanoparticles and polymers, will enable the creation of hybrid nanostructures with synergistic properties. Furthermore, the exploration of CDs for advanced applications, such as theranostics, energy storage, and catalysis, will continue to be a key area of investigation.
Further Reading
Nanomaterials, A Review on Carbon Dots: Synthesis, Characterization and Its Application in Optical Sensor for Environmental Monitoring
ACS Central Science, Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications
