Fullerenes (Buckyballs): Nanotechnology Definition, Uses, Properties
Definition: Fullerene, commonly known as buckyball, is a form of carbon molecule that resembles a soccer ball, composed of carbon atoms linked in hexagonal and pentagonal shapes. This article explores the structure, properties, synthesis, applications, and challenges associated with fullerenes, particularly focusing on the spherical variety known as buckyballs.
Discovery and Nobel Recognition
The discovery of fullerenes, specifically the C60 molecule known as the buckyball, was a groundbreaking event in materials science. In 1985, Richard Smalley, Robert Curl, and Harold Kroto discovered C60 while experimenting with carbon clusters, aiming to replicate the conditions of the interstellar medium. They identified a molecule consisting of 60 carbon atoms arranged in a spherical shape reminiscent of a soccer ball or a geodesic dome, a design associated with architect Buckminster Fuller, hence the name "buckminsterfullerene."
This discovery was initially unexpected and expanded the known forms of carbon beyond graphite and diamond, introducing a new class of carbon allotropes. The unique stability and symmetry of C60, along with its potential applications in various fields, underscored the significance of this new carbon form. In recognition of their pioneering work and its impact on chemistry and materials science, Smalley, Curl, and Kroto were awarded the Nobel Prize in Chemistry in 1996. The Nobel Committee noted that the discovery of fullerenes had not only unveiled a new form of carbon but also opened up new research avenues in nanotechnology and materials science, highlighting the molecule's broad implications for future technological applications.
Structure and Composition
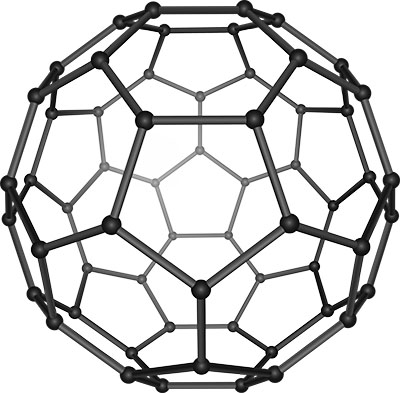
The most well-known fullerene is C60, consisting of 60 carbon atoms arranged in a structure that mirrors a soccer ball, with 20 hexagons and 12 pentagons. No two pentagons share an edge, which minimizes the strain on the spherical configuration. This geometric arrangement grants fullerenes their unique physical and chemical properties.
Beyond C60, fullerenes can vary in size and structure, with C72, C84, and even larger molecules being synthesized. C72 and C84 fullerenes maintain the soccer ball-like structure but with additional hexagons that increase the molecule's size and alter its properties
Fullerenes larger than C60, such as C72, C84, and others, are part of the fullerene family but are not typically referred to as "buckyballs." The term "buckyball" is specifically associated with C60 fullerenes due to their spherical shape resembling a soccer ball or a geodesic dome, which was designed by architect Buckminster Fuller. The name "buckyball" is a homage to Fuller, and it specifically denotes the spherical C60 molecule.
- C72 Fullerene: Features a more elongated shape compared to C60, due to its 12 extra carbon atoms. This alteration in structure affects its electronic properties, making it less symmetric and potentially altering its reactivity and stability.
- C84 Fullerene: This fullerene has even more variations in structure due to its increased size, allowing for multiple isomers. The structure of C84 fullerenes can significantly influence their electronic and physical properties, offering a wider range of potential applications and behaviors. The diversity in C84 isomers makes them a subject of interest for research in molecular electronics and photonics.
These variations in structure among C60, C72, and C84 fullerenes lead to differences in their physical and chemical properties, affecting their potential applications in nanotechnology, electronics, and materials science.
Synthesis of Fullerenes
Fullerenes are synthesized through methods such as arc discharge in an inert atmosphere, laser ablation of carbon, or chemical vapor deposition. These processes generate a soot that contains fullerenes, which are then separated and purified for various applications.
Unique Properties
Fullerenes exhibit remarkable properties, including:
- High Stability: The unique carbon structure gives fullerenes considerable stability and the ability to withstand high pressures and temperatures.
- Electron Affinity: Fullerenes can accept electrons, making them excellent electron acceptors. This property is beneficial in applications such as organic photovoltaics.
- Chemical Versatility: They can participate in a variety of chemical reactions, allowing the creation of many different fullerene derivatives with tailored properties.
Applications of Fullerenes
Fullerenes, with their unique carbon-cage structures, have found applications across a diverse range of fields. These applications exploit the exceptional properties of fullerenes, such as their ability to act as superconductors, their photovoltaic properties, and their potential in medical therapies. Below are specific examples illustrating the versatility of fullerenes in various domains:
Medicine and Health
- Drug Delivery Systems: Fullerenes have been explored as carriers for drug delivery due to their ability to penetrate biological membranes and their capacity to be functionalized with various therapeutic molecules. For instance, C60 fullerenes can be modified to transport anti-cancer drugs directly to tumor cells, minimizing the impact on healthy tissues and reducing side effects.
- Antioxidants: The radical scavenging properties of fullerenes are utilized in creating powerful antioxidants. C60 fullerene, in particular, has been found to have an exceptionally high affinity for free radicals, making it a potent ingredient in anti-aging skin creams and potentially in treatments to mitigate oxidative stress-related diseases.
Energy and Environmental Applications
- Photovoltaics: Fullerenes are integral to the development of organic photovoltaic cells (OPVs). They serve as electron acceptors, enhancing the efficiency of OPVs by facilitating electron transfer and increasing the absorption of light. This application showcases their potential in renewable energy technologies.
- Environmental Cleanup: The unique properties of fullerenes have been harnessed for environmental remediation, such as in the cleanup of water contaminants. Fullerenes can absorb harmful compounds, making them useful in filtering and purifying processes to remove pollutants from water bodies.
Electronics and Materials Science
- Superconductors: Certain fullerene compounds, when combined with alkali metals, exhibit superconductivity at relatively high temperatures. This has implications for the development of superconducting materials for electronic devices, potentially leading to more efficient power transmission technologies.
- Composite Materials: The incorporation of fullerenes into composite materials can significantly enhance their strength, thermal stability, and resistance to wear. This application is particularly valuable in the aerospace and automotive industries, where material performance is critical.
Cosmetics and Consumer Products
Leveraging the antioxidant properties of fullerenes, the cosmetic industry has developed skincare products that promise to protect the skin from UV radiation, reduce the appearance of wrinkles, and prevent premature aging by neutralizing free radicals.
Scientific Research
Fullerenes have been explored as qubits in quantum computing due to their stable electronic properties and the ability to encapsulate atoms or molecules, which can be manipulated to represent quantum bits. This application is still in the experimental stage but highlights the potential of fullerenes in next-generation computing technologies.
Challenges and Future Perspectives
Despite their potential, fullerenes face challenges such as high production costs and the need for further research to fully understand their environmental impact. Ongoing research aims to overcome these challenges and expand their applications in various fields.
Conclusion
Fullerenes, especially buckyballs, are a fascinating aspect of nanotechnology, offering unique properties and applications. As research advances, their role in various industries is expected to grow, highlighting their importance in the development of nanomaterials and nanotechnology.
Further Reading
ECS Journal of Solid State Science and Technology, Review???The Beautiful Molecule: 30 Years of C60 and Its Derivatives
Journal of Indian Academy of Oral Medicine and Radiology, Fullerene and its applications - A review
