| Jun 05, 2019 | |
How to characterize nanoparticles |
|
| (Nanowerk Spotlight) Currently, a key issue hindering the utility of nanoparticles in more sophisticated applications is reproducibility. This problem is, however, partially intrinsic, as the product of synthesis is always prone to yield a polydispersion of nanoparticles, sometimes with a broad distribution of sizes, shapes, and defects. | |
| This makes nanoparticle characterization a crucial step required to fully comprehend the origin of nanoparticle behavior, and subsequently translate their performance benefits from laboratories into specific real word applications. | |
| There is at the same time a need to establish standardized, well-characterized nanoparticles that vary in size, shape, durability, composition and surface reactivity for use in metrology, characterisation, exposure and hazard assessment (notably for comparative benchmarking purposes in toxicology). | |
| Researchers are still challenged by the task of determining the physicochemical properties of nanoparticles and exploring their structure–function relationships. A key limitation is their ability to fully investigate the nanoscale realm: Different characterization techniques are based on different physical properties, therefore only providing a partial picture of the nanoparticle characteristics. | |
| Making matters more challenging yet, the characterization methods themselves can directly affect the measured quantities of nanoparticles. | |
| Nanoparticle characterization is a broad and complex discipline. However, measurement and standardization often lag behind the rapid development of new nanomaterials and their applications. Furthermore, the demands on reproducibility and quality control increase steadily as new materials leave the discovery stage and move into commercial applications. | |
| Nanoparticles exist in various chemical compositions ranging from micelles to metal(oxide)s, from synthetic polymers to large biomolecules. Each of these materials features a completely different chemistry, which can be analyzed by a variety of methods including optical spectroscopy, X-ray fluorescence and absorbance, Raman spectroscopy, and solid-state NMR. | |
| However, often the behavior of nanoparticles is largely governed by their nanometer dimensions. As such, throughout nanoparticle characterization, the investigation of size, shape, surface charge and porosity is a fundamental step for fully understanding and predicting their behavior. | |
| These essential parameters are the focus of a review article in Advanced Materials ("Nanoparticle Characterization: What to Measure?"). It provides a set of guidelines to investigate and characterize the key parameters defining a nanoparticle sample, namely size, shape, surface charge, and porosity. | |
| First, the authors define these physicochemical terms and their implication in affecting nanoparticle properties. They then provide a critical overview of established and specialized techniques currently used for evaluation of nanoparticles, and discuss their practical advantages and disadvantages. | |
| Concluding their review, the authors propose some reasonable recommendations of how the physicochemical parameters of nanoparticles should be investigated, and how to characterize these key properties in different environments according to the intended nanoparticle use. | |
Nanoparticle parameters |
|
| The ability to be dispersed into discrete entities, each characterized by a size, shape, surface charge and porosity discriminate nanoparticles from the wider class of nanomaterials encompassing nanostructured objects that might present dimensions in the micro and millimeter regime, such as nanostructured films or nanotubes. | |
| The review provides a description of these physicochemical terms in the context of nanoparticle technology, and the different physical and experimental means in which these parameters can be defined. | |
| Size and shape affect the nanoparticle functionalization capacity, fluid drag and diffusion, optical properties, and uptake into cells. | |
 |
|
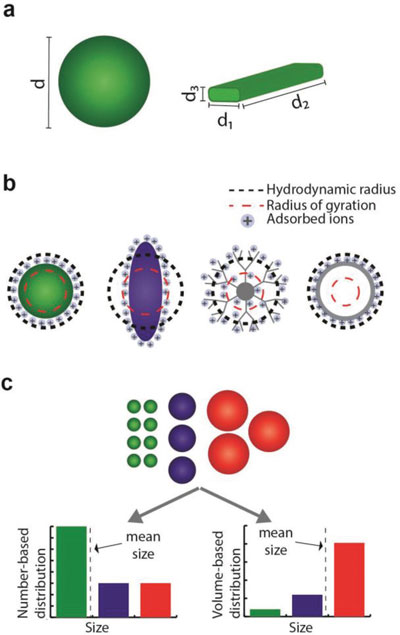
| Nanoparticle size characterization. a) Spherical particles are described by a single size parameter. However, for nonspherical nanoparticles, several dimensions are needed to fully report their dimensions; b) the calculation of the effective radius is based on nanoparticle behavior or on the method of detection. The definition of this quantity might differ considerably from the physical nanoparticle dimensions; c) different mean sizes can be calculated for a nanoparticle population according to the weighting factors assigned to the population components. (Reprinted with permission by Wiley-VCH Verlag) | |
 |
|
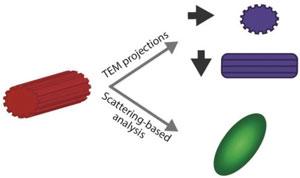
| Nanoparticle shape characterization. TEM provides nanometer resolution on nanoparticle morphology. However, the measured shape is a 2D projection of the nanoparticle morphology, and therefore depends on the relative orientation of the nanoparticle and the electron beam. Scattering-based techniques provide qualitative information on the nanoparticle shape. (Reprinted with permission by Wiley-VCH Verlag) | |
| Surface charge, besides controlling the stability of a colloidal suspension and its tendency toward aggregation, also plays a major role in shaping the interactions between nanoparticles and the environment. | |
 |
|
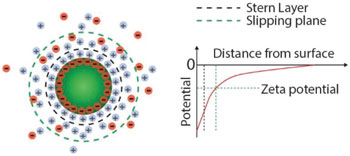
| Surface charge and zeta potential of nanoparticles in suspension. Charges on the nanoparticle surface are screened by the free ions in solution, giving rise to two ion layers: a first layer of adsorbed ions on the nanoparticle surface, the so-called Stern layer; a second layer of stationary but diffusing ions that move with the particle. The zeta potential is defined as the potential difference between the slipping plane, i.e., the plane that “separates” the cloud of stationary ions around the particles from freely diffusing ions in solution, and the potential of the bulk solution. (Reprinted with permission by Wiley-VCH Verlag) | |
| Finally, owing to their increased surface-to-volume ratio, nanoparticles possess a large external surface area that can be functionalized for different applications. | |
| In addition, porous or hollow nanoparticles also exhibit a vast internal surface area, which can be further functionalized to impart additional functionalities, such as the design of smart nanoparticle drug delivery systems. The possibility of synthesizing nanoparticles featuring porous frameworks – such as metal-organic frameworks (MOFs) – has greatly expanded the range of application of nanomaterials. Porosity provides the nanoparticles with a drastic increase in their surface-to-volume ratio, which can exceed that of solid particles with equal dimensions by several orders of magnitude. | |
Characterization methods |
|
| Several characterization methods have been devised to investigate size, distribution, shape, surface charge and porosity of nanoparticles in different environments. In their review, the authors discuss the main techniques for the characterization of these key parameters both in the dry state and in solution. | |
| They also introduce some specialized techniques for nanoparticle characterization, which enable to expand the accessible range of information to gain deeper insights into specific nanoparticle properties. | |
| Given the large number of existing approaches and techniques, including the combination of different methods, different variations of the same techniques, and different approaches to data analysis for a same technique, the authors caution that their review cannot provide an exhaustive list of all available methods for nanoparticle characterization. Rather, they provide a selection of methods that in their opinion are best suited to characterize a broad range of nanomaterials, which are commonly used and well established: | |
| Characterization in dry state | |
|
|
|
| Characterization in suspension | |
|
|
|
| The review also presents advanced techniques that are of interest for the characterization of specific properties of nanoparticles and of their behavior, which cannot be accessed using standard characterization methods: | |
|
|
|
Nanoparticle characterization overview |
|
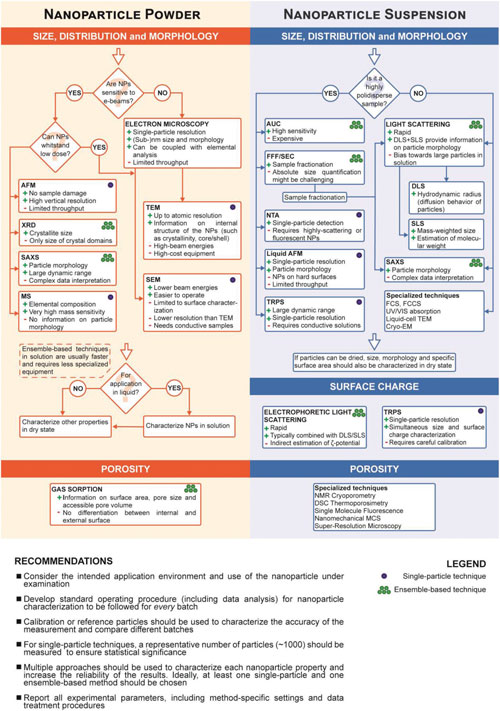
| In order to present the reader with some general recommendations to help with the selection of methods in a wide range of applications, the authors provide a framework organizing the most important techniques described in their article into groups and key questions to help the reader decide which techniques are most suitable for the sample under investigation. | |
 |
|
| Flowchart depicting the main steps for the characterization of size, size distribution, morphology, surface charge, and porosity of nanoparticles (NP), both in dry state and in solution. (click on image to enlarge) | |
| Although it is not possible to provide a comprehensive overview of all techniques for all types of samples, this workflow can be applied to many typical cases of nanomaterials. | |
| To be able to correlate the physicochemical properties of the nanoparticles with their performance in a specific task, characterization needs to be both accurate and precise. Therefore, as the authors recommend, standardized standard operating procedures should be developed to improve the comparability of results between different materials and laboratories: "It is crucial that new data can be compared and evaluated with previously published results in a meaningful way. This will require conclusive and harmonized analytical protocols to be applied worldwide." | |
| Concluding their review, the authors make the following general recommendations for nanoparticle characterization: | |
| 1. Consider the intended form the target application of the nanoparticles. The form (colloidal suspension or solid powder) often dictates the need for the specific characterizations that should be used. | |
| 2. Place reasonable bounds on the precision and statistical confidence with which physicochemical properties need to be measured to satisfy the needs of the application. For example, the statistical demands in the quality control for pharmaceutical formulations will typically be higher than for industrial coating materials. | |
| 3. Develop or adopt standard characterization and standard operating procedures to be systematically and thoroughly followed for every batch. | |
| 4. The protocol used to measure the physicochemical parameters as well as the meta data should be described in detail. Besides a detailed description of the experimental protocol, the experimental parameters, and method-specific settings that were used, this should include how the data were analyzed (e.g., what model was used to obtain a certain value). | |
| 5. In the case of suspensions, the exact chemical composition of the liquid matrix should be specified. Both pH and ionic strength should be measured and reported for aqueous solutions. | |
| 6. Measured parameters should be calibrated against a standardized reference. If no standardized references for a quantitative comparison with the nanomaterial at hand are available, internal references can be used to, at least, ensure consistency between batches. For example, obtaining similar size distributions from different nanoparticle batches and/or a constant reference sample should ensure the reproducibility of results. | |
| 7. Whenever feasible, more than one technique should be used to characterize the same quantity. | |
| 8. The obtained results should be compared with published data whenever feasible. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
