What is a MOF (metal organic framework)?
Metal-organic frameworks (MOFs) are organic-inorganic hybrid crystalline porous materials that consist of a regular array of positively charged metal ions surrounded by organic 'linker' molecules. The metal ions form nodes that bind the arms of the linkers together to form a repeating, cage-like structure. Due to this hollow structure, MOFs have an extraordinarily large internal surface area.
Researchers have synthesized MOFs that feature a surface area of more than 7800 square meters per gram. To put this into context, if you could lay out the available surface area in a teaspoon of this material (around a gram of solid), it would cover an entire soccer field.
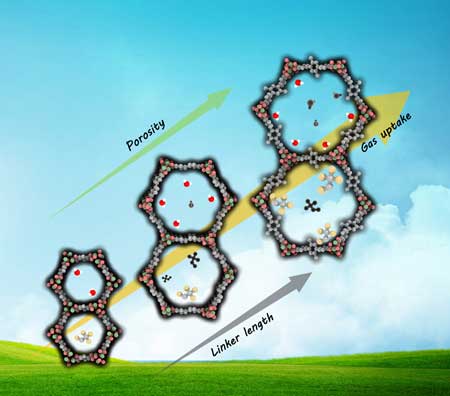
MOFs offer unique structural diversity in contrast to other porous materials – uniform pore structures; atomic-level structural uniformity; tunable porosity; extensive varieties; and flexibility in network topology, geometry, dimension, and chemical functionality. This allows researchers the successful control of framework topology, porosity, and functionality.
MOFs unique structure design and tunability – crystalline porous materials that are composed of both organic and inorganic components in a rigid periodic networked structure – is not readily accessible in conventional porous materials, e.g., purely inorganic zeolites.
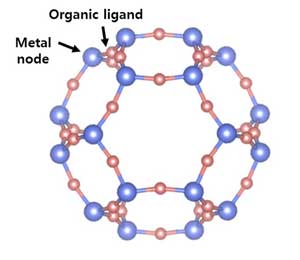
By making the MOF from different metal atoms and organic linkers, researchers can create materials that selectively absorb specific gases into tailor-made pockets within the structure. MOFs therefore offer great potential for their effective integration and exploration in various sensing applications.
MOFs can be put together arbitrarily like Lego bricks and outperform every previously known class of material in terms of flexibility.
History and Background
The physicochemical properties of materials are governed by the synergistic effects of structures and compositions, and MOFs are fascinating examples of how the unique structure of hollow-structured materials can provide a whole raft of advantageous features. Among them are enhanced surface-to-volume ratio; low density; microreactor environment; higher loading capacities; and reduced transmission lengths of mass and charge.
Consequently, the preparation of hollow structures for technological applications has long been a popular research field for chemists and materials scientists. However, the synthesis of porous or hollow-structured materials with controllable – and especially complex – structures and certain composition in a controlled manner has always been a challenge for scientists.
Enter MOFs – crystalline hybrid materials created from both organic and inorganic molecules via molecular self-assembly. Pioneered in the late 1990s ("Design and synthesis of an exceptionally stable and highly porous metal-organic framework") by Prof. Omar Yaghi at UC Berkeley, MOFs have become a rapidly growing research field.
So far, more than 90 000 different MOF structures have been reported and the number grows daily.
Though exciting, the sheer number of MOFs is actually creating a problem: It researchers propose to synthesize a new MOF, how can they know if it is truly a new structure and not some minor variation of a structure that has already been synthesized? To address the issue, researchers are using machine learning to develop a 'language' for comparing two materials and quantifying the differences between them (read more: "Machine-learning helps sort out massive MOF materials' databases").
MOF Applications
Numerous applications in many fields are being developed that exploit MOFs' cage-like structure, such as gas storage and separation, liquid separation and purification, electrochemical energy storage, catalysis, and sensing.
In addition to direct applications, MOFs have been used as unique precursors for the construction of inorganic functional materials with unparalleled design possibilities, such as carbons, metal-based compounds, and their composites.
Currently, carbonaceous materials are attracting much interest for their extensive applications including adsorption, catalysis, batteries, fuel cells, supercapacitors, and drug delivery and imaging. In addition, some sensors are also one of the important applications of carbonaceous materials, because they are closely related to human health.
There are varieties of approaches for the preparation of these carbon materials, but among them, directly carbonizing from organic precursors is the most frequently used method to prepare nanoporous carbons due to its flexibility and simplicity. These materials present certain drawbacks, though, such as low surface areas, disordered structures, and non-uniform sizes, which will greatly limit their applications.
However, researchers found that carbon materials derived from metal-organic frameworks (MOFs) could overcome these limitations (read more: "Applications of carbon materials derived from metal-organic frameworks").
MOF Gas Sensors
Typically, the instruments that can detect traces of a specific gas in the air are large, expensive, energy-intensive machines.
One promising way to make small, inexpensive, and energy-efficient gas sensors involves porous materials like metal-organic frameworks (MOFs). By making the MOF from different metal atoms and organic linkers, researchers can create materials that selectively absorb specific gases into tailor-made pockets within the structure.
MOFs' high surface area also is a beneficial aspect for high-performance gas sensors.
One example is a thin-film a tailor-made MOF, coated onto an electrode, that forms an electronic sensor that could detect traces of sulfur dioxide gas.
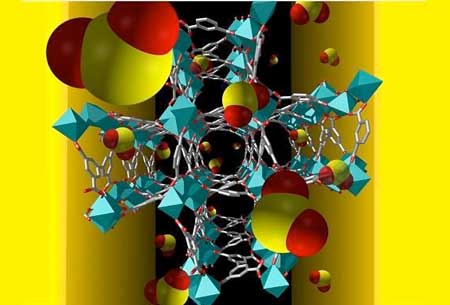
Scientists also found that a metal–organic framework, MFM-300(Al), not only effectively filters harmful nitrogen dioxide gas, but it also has outstanding capabilities for ammonia storage.
Carbon Capture
One particular MOF material exhibits an unprecedented cooperative mechanism for carbon dioxide capture-and-release with only small shifts in temperature. This structure of the MOF, with CO2 adsorbed, closely resembles the RuBisCO enzyme found in plants, which captures CO2 from the atmosphere for conversion into nutrients.
The discovery paves the way for designing more efficient materials that dramatically reduce overall energy cost of carbon capture. Such materials could be used for carbon capture from fossil-fuel-based power plants as well as from the atmosphere, mitigating the greenhouse effect.
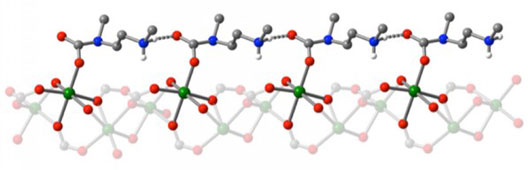
In other research, Mg-MOF-74, an open metal site MOF, has emerged as one of the most promising strategies for capturing and storing greenhouse gases.
MOFs as biomedical microrobots
By applying concepts developed in micro- and nanorobotics, researchers have demonstrated the controlled motion and delivery of cargo payloads embedded in MOFs. These helical MOF-based micromachines, termed MOFBOTs, are propelled by artificial bacterial flagella, can swim and follow complex trajectories in three dimensions under the control of weak rotational magnetic fields.
MOFs for Refrigeration
Similar to the carbon capture application described above, researchers are exploring ways how MOFs may help lower energy consumption for air conditioning by engineering them to hold onto a large amount of refrigerant gases.
The high attachment of this gas – an environmentally friendly fluorocarbon called R134 and water – to MOFs hold promise for their use in adsorbent cooling systems that a can be powered by waste heat.
And the small nanostructure of the MOF and its higher sorption rate means the cooling systems can be made much smaller and, therefore, more efficient and economically viable.
Another approach to cooling and heating involves MOF coatings that absorb water vapor like chillers or heat pumps.
Removing heavy metals from water with MOFs
Researchers treated a MOF, known as Fe-BTC, with dopamine, which polymerized to polydopamine (PDA) pinning the polymer inside the MOF. The final composite, named Fe-BTC/PDA, can quickly and selectively remove high amounts of heavy metals like lead and mercury from water samples. In fact, it can remove over 1.6 times its own weight of mercury and 0.4 times of its weight of lead.
Fe-BTC/PDA was then tested in solutions as toxic as some of the worst water samples found in Flint, Michigan. The tests showed that the MOF can, in a matter of seconds, reduce lead concentrations to 2 parts per billion, a level that the U.S. Environmental Protection Agency and World Health Organization deem drinkable.
MOFs to capture nuclear waste
At nuclear power plants and legacy waste sites, a particularly difficult-to-capture hazard is radioactive organic iodides. These compounds are made of hydrocarbons and iodine. By chemically modifying MOFs with binding sites that have reactive nitrogen that can bind to organic iodides, scientists have built MOF traps that exhibit a high methyl iodide capacity – over three times higher than the currently used industrial adsorbent under identical conditions.
Also, these new MOFs advantageously serve as good absorbents at lower temperatures. Furthermore, the MOF adsorbent can be recycled multiple times without loss of capacity, unlike other known industrial absorbents.
MOF Vaccines
MOF vaccines are based on a biocompatible polymer framework that 'freezes' proteins inside vaccines. The proteins then dissolve when injected in human skin. This innovation could help health care providers transport and administer vaccines in remote areas with unreliable power.
MOF vaccines are crystals that contain an antigen like the protein on the surface of influenza, except that since they’re frozen inside a crystalline lattice, they can’t denature or change shape.
Structural advantages of MOFs allow them to perform better at room temperature than artificial encasings like silica. Specifically, MOFs’ porous structure allows them to function as a semipermeable barrier to transport biological matter like proteins or antigens in vaccines.
Implantable MOF Nutrient Sensors
By integrating MOFs with flexible electronics, the electrochemical detection of nutrients without using enzymes becomes possible. In proof-of-concept work, researchers already have demonstrated MOF sensors that can be used to detect trace of ascorbic acid, L-Tryptophan, glycine, and glucose, all of which are nutriments that are closely involved in the metabolism and circulation processes.
These sensor can be implanted and, as MOFs are very stable, the new technique could potentially be used to conduct long-term monitoring of biomolecules at different locations simultaneously.
These devices could be used as a tool to help better understand various life processes. They can be used as implants to monitor biomolecules at different locations of various organs. When integrated with more stimulation and measurement functions, this type of devices can be used to control animal behaviors, reveal underlying mechanism of biological processes, monitor health conditions, and treat diseases.
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
