| Feb 20, 2019 | |
Applications of carbon materials derived from metal-organic frameworks (MOFs) |
|
| (Nanowerk Spotlight) Electrochemical energy storage devices provide a promising approach for the storage of electric energy from renewable – yet intermittent – sources such as wind, sunlight, biofuels, hydroelectricity, geothermal heat, etc. | |
| Currently, carbonaceous materials are attracting much interest for their extensive applications including adsorption, catalysis, batteries, fuel cells, supercapacitors, and drug delivery and imaging. In addition, some sensors are also one of the important applications of carbonaceous materials, because they are closely related to human health. | |
| There are varieties of approaches for the preparation of carbon materials, such as directly carbonizing from organic precursors, physically or chemically carbonizing from carbon, template methods using zeolites and mesoporous silica, solvothermal and hydrothermal methods with elevated temperature, the electrical arc methods, and chemical vapor decomposition (CVD) methods. | |
| Among all these approaches, directly carbonizing from organic precursors is the most frequently used method to prepare nanoporous carbons due to its flexibility and simplicity. These materials present certain drawbacks, though, such as low surface areas, disordered structures, and non-uniform sizes, which will greatly limit their applications. | |
| However, researchers found that carbon materials derived from metal-organic frameworks (MOFs) could overcome these limitations (read our primer on Metal-organic frameworks). | |
| MOFs are organic-inorganic hybrid crystalline porous materials that are composed of single metal ions or metal clusters linked by polytopic organic ligands. MOFs offer unique structural diversity in contrast to other porous materials, allowing the successful control of framework topology, porosity, and functionality. | |
| MOF-derived carbon materials maintain various advantages originating from their MOFs, such as high porosity (up to 90%), extremely high surface area (almost 10 000 m2 g-1), tunable pore sizes, and outstanding cycle lifetime. | |
| As a result, MOF-derived carbon materials have shown promising applications including adsorption, energy storage and conversion, gas storage and separation, catalysis, chemical sensing, and solid phase extraction. | |
| A review article published in Advanced Materials ("Applications of Metal–Organic-Framework-Derived Carbon Materials") summarizes the applications of MOF-derived carbon materials. | |
| The authors focus on applications in batteries, supercapacitors, electrocatalytic reactions including oxygen reduction reactions, oxygen evolution reactions and hydrogen evolution reactions,and water treatment. | |
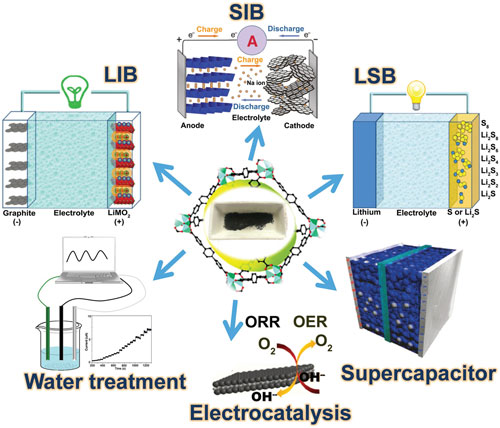 |
|
| MOF-derived carbon materials and their promising applications. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
Metal–Organic-Framework-Derived Carbon Materials for Batteries |
|
| In order to improve the electrochemical activities of batteries, researchers also designed and investigated carbon materials derived from MOFs. As the controlling morphology of electrode materials in fabrication process is of great significance to the chemical performance of a battery, the review lists these applications in different kinds of batteries (lithium-ion batteries, lithium–sulfur batteries, sodium-ion batteries, and other kinds of batteries such as lithium–selenium batteries) according to their morphology of carbonaceous materials in electrodes. | |
| This section includes a detailed discussion of the various carbon materials that are being developed for batteries: normal porous carbon; carbon nanosheets; carbon nanocages; and other morphologies such as cuboids and nanorods. | |
| In reviewing the literature, the authors draw the conclusion that already a considerable number of MOF-derived carbon materials have been applied to batteries by controlling the carbonization temperature, adjusting various morphologies and varying reaction conditions. | |
Metal–Organic-Framework-Derived Carbon Materials for Supercapacitors |
|
| Electrochemical capacitors, also acknowledged as supercapacitors, are electrochemical energy storage devices that provide higher power density than batteries and higher energy density than conventional dielectric capacitors. | |
| Supercapacitors possess great advantages of rapid charge and discharge and highly prolonged cycle life. However, they may suffer from low energy density compared to batteries. Supercapacitors may be much more complicated devices when using different types of electrodes, since composite electrodes can perform both capacitive and faradaic charge storage. | |
| This section of the review summarizes some typical supercapacitors classified by their electrode materials: nanoporous carbon; metal oxide/carbon composites; and other nanocomposite materials. | |
Metal–Organic-Framework-Derived Carbon Materials for Electrocatalytic Reactions |
|
| Though vast advancements have been made in the design and application of MOF-based materials, it was not until recently that their potential applications in the field of electrochemical catalysis drew the attention of researchers. | |
| Fuel cells, particularly H2/O2 or H2/air batteries, have become promising candidates to provide energy for vehicles and convert H2 (with O2 or air) into energy produced by intermittent renewable sources such as sunlight, wind and waves. | |
| OER is the core step of anodic reaction, where the H2O in the solvent is oxidized to O2, and ORR is the main reaction of cathode reaction, in which O2 is reduced to produce H2O2 via a 2- electron pathway, or H2O through a 4- electron pathway. | |
| The review focuses on the application of MOF-based materials as catalysts in these important energy-conversion electrochemical reactions, including the oxygen reduction reaction, oxygen evolution reaction and hydrogen evolution reaction. | |
Metal–Organic-Framework-Derived Carbon Materials for Water Treatments |
|
| Many efforts have been made to reduce and/or remove environmental pollutants, and a large number of environmental pollution treatment technologies have emerged, such as biological treatments, chemical oxidation, adsorption and membrane separation methods, etc. | |
| Among them, the adsorption method has always been favored. The advantages of high specific surface area, high and adjustable porosity, diversity of structural composition, open metal sites, and chemical modification allow MOF-derived carbon materials (in particular porous carbon derived from ZIF-8) to be superior materials in the application of adsorption, thanks to their increased active sites. | |
| Concluding their review, the authors provide their perspectives of some of the key challenges in this field: | |
| 1) The preparation route of most MOF-derived carbon materials is depended on high-temperature calcination. However, calcination usually destroys the ordered structure and porous morphology of MOFs, and even leads to polymerization, which weakens the catalytic activity. Therefore, a low reaction temperature is generally preferred. | |
| 2) Most of the studies on MOF-derived carbon materials are still focused on the preparation and characterization, but the research on their mechanisms in reactions is not very mature. | |
| A thorough understanding and mastery of the interfacial interactions between MOFs and the additional components are greatly in need since they could provide guidance to optimize the synthetic process of MOF-derived carbon materials for better performance. | |
| 3) Although some strategies have been recommended to enhance the firmness between the MOF film and the carrier, such as spin-on polymer adhesives or alkyl functionalized carriers, they do not have commercial value. Therefore, a universal, low cost, and easy operating strategy is needed. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
