| Feb 05, 2020 | |
How manipulating ligand interactions in metal clusters can spur advances in nanotechnology(Nanowerk News) When metal atoms form small clusters of a particular size, they show interesting and potentially useful electromagnetic characteristics, which are different from those of the actual bulk metal. To fully explore the potential of these properties, it is necessary to find ways to assemble precise macroscopic structures out of these clusters. |
|
| But, how do these clusters bind together, and what exactly dictates their properties? These questions have remained unanswered, until now. | |
| In a new study published in Materials Horizons ("Understanding and designing one-dimensional assemblies of ligand-protected metal nanoclusters"), researchers from the Tokyo University of Science, led by Prof Yuichi Negishi, set out to find these answers. | |
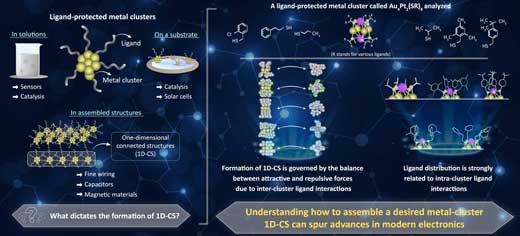
| Prof Negishi explains the motivation behind this study, "Previous studies have found that gold clusters can form one-dimensional connected structures (1D-CS) that are linked via a single gold atom in each cluster. While assembling ligand-protected metal clusters is an interesting approach to realize new physical properties and functions, the factors required for the formation of 1D-CS are currently poorly understood." | |
 |
|
| Interactions among ligands dictate the final structure of metal clusters, which have various applications in modern electronic devices. (Image: Tokyo University of Science) (click on image to enlarge) | |
| To begin with, the researchers wanted to see how intra-cluster ligand interactions dictate the formation of metal clusters. For this, they focused on a particular type of ligand-protected metal cluster called "thiolate (SR)-protected gold-platinum alloy cluster ([Au4Pt2(SR)8]0)", as it had various types of ligand distributions. | |
| Through techniques like single crystal X-ray structural analysis, the researchers found that these metal clusters attach to one another via gold atom bonds, and these bonds form 1D-CS depending on the attractive and repulsive forces caused by inter-cluster ligand interactions. | |
| They also found that these interactions are affected by how ligands are distributed on the clusters and the angles that they form. More specifically, when ligands were uniformly spread around the metal cluster (which indicates repulsive forces between ligands), the repulsive forces between different metal clusters were also higher, thereby preventing the formation of 1D-CS. | |
| Prof Negishi explains, "We found that the ligand distribution in [Au4Pt2(SR)8]0 changes depending on the ligand structure and that differences in the distributions of the ligands influence the inter-cluster ligand interactions. Thus, we need to design intra-cluster ligand interactions to produce 1D-CS with desired connecting structures." | |
| In fact, through further analyses, the scientists even found that the formation of 1D-CS had an effect on the overall electronic structure of the metal clusters, even affecting their conductivity. | |
| These findings serve as guidelines for those trying to create 1D-CS to leverage the potential of metal cluster assemblies. One notable application of metal clusters, states Prof Negishi, is the fabrication of finer wiring. He says, "It is challenging to draw finer wiring using conventional top-down technology; fortunately, progress in the field of metal nanoclusters will enable the development of bottom-up technology for drawing finer wiring." | |
| This study paves the way for significant improvements in sophisticated electronics systems and devices. Not just this, these findings will act as a beacon for scientists working in the fields of nanotechnology and nanoengineering. |
| Source: Tokyo University of Science | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
