| Dec 22, 2020 |
Transforming self-assembled architectures into functional materials
(Nanowerk News) Imagine if a material would arrange itself into a shape suited for its application. It may result in a catalyst that maximizes its own surface area for improved efficiency or a micro-actuator that forms appendages to grab nearby objects. This is the promise that self-assembly holds: making complex, functional materials by letting matter shape itself.
|
|
Yet, not all matter that self-assembles into interesting forms turns out to have a useful function in its final shape. Researchers of the Self-Organizing Matter group recently discovered that ion exchange allows them to separate the self-assembly process from the resulting material.
|
|
Their findings were published in Advanced Materials ("Shape-Preserving Chemical Conversion of Architected Nanocomposites").
|
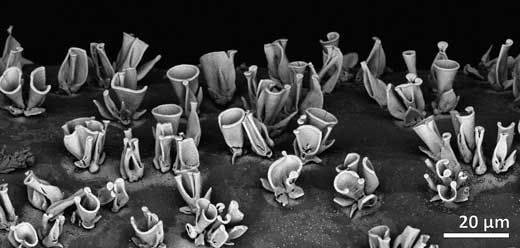 |
| A field of converted nanocomposites, their shape formed with self-assembly and their composition tuned with conversion reactions. (Image: AMOLF) (click on image to enlarge)
|
|
With their beautiful and intricate shapes the nanocomposites studied by the Self-Organizing Matter group look quite remarkable (see illustration). Yet, PhD students Hans Hendrikse and Arno van der Weijden wanted more than beautiful structures and had an itch to also utilize the functionality of the nanocomposites.
|
|
Encouraged by the shapeability and structural layout of their nanocomposites, they started investigating the options together with researchers from the University of Amsterdam, ARNCL, Leiden University and Virginia Tech.
|
|
The research team started off with nanocomposites that consisted of barium carbonate (BaCO3) nanocrystals embedded in a silica (SiO2) matrix and converted these to cadmium sulphide (CdS).
|
|
First, they established a route to reproducibly convert the nanocomposites to this final material, while investigating the properties of the nanocomposites during ion exchange. Through analysis with electron microscopy and x-ray diffraction the team learned something fascinating: the small size of the BaCO3 nanocrystals made them exceptionally susceptible to ion exchange reactions, while the surrounding SiO2 matrix provided mechanical stability to maintain the original nanocomposite’s shape during conversion.
|
|
Hans Hendrikse says, “it is almost like we are changing out some of the bricks of a house while keeping the overall structure intact.”
|
|
Based on these insights, expanding the selection of materials was straightforward and new routes were developed to change the nanocomposite’s composition to various cadmium, iron, nickel and manganese salts. Moreover, the original nanocomposite can be shaped in a large selection of pre-determined shapes.
|
|
All these shapes can be converted to any of the above mentioned compositions. So not only is it possible to convert nanocomposites, there is also a variety of materials and shapes to interchangeably choose from.
|
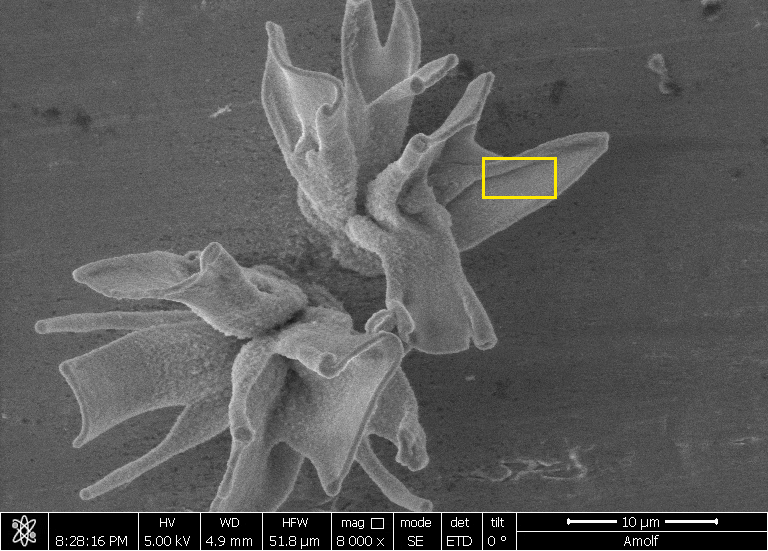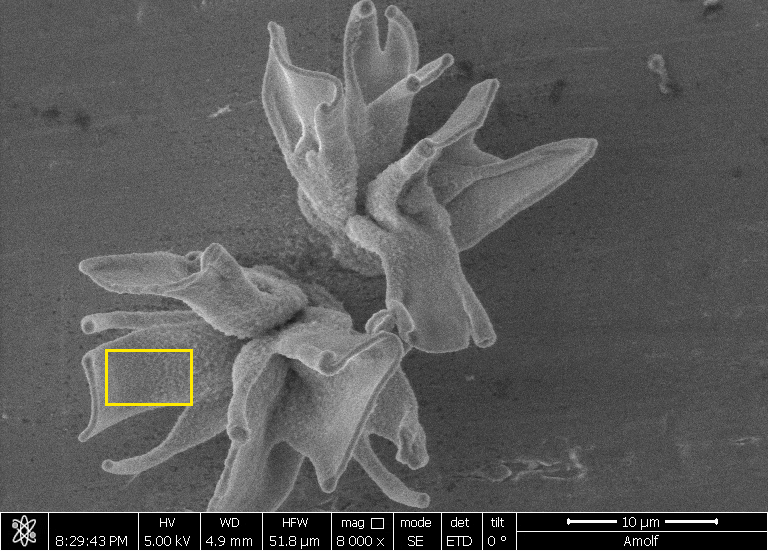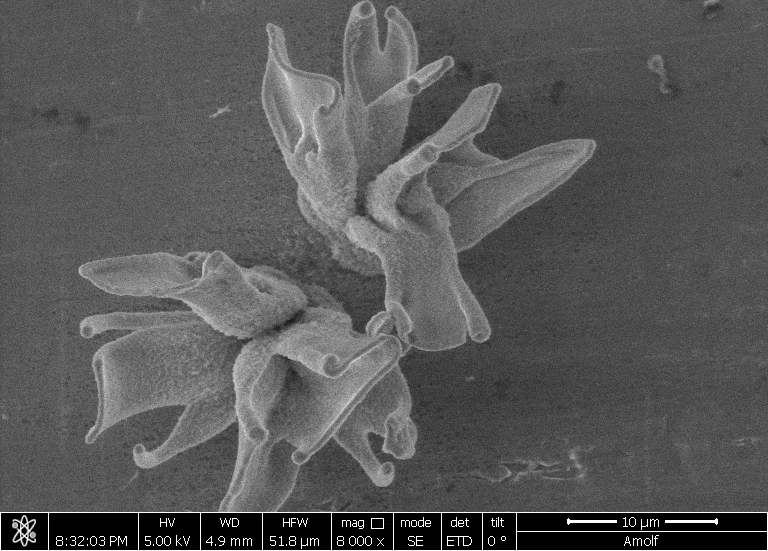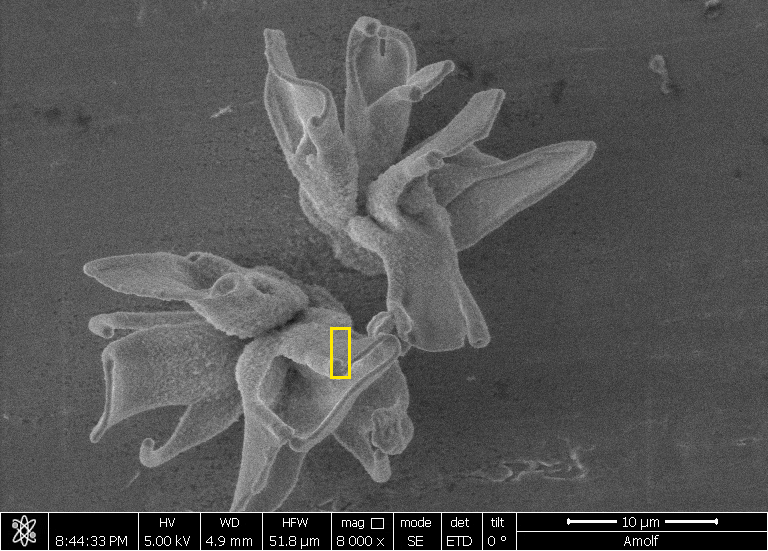 |
| A field of converted nanocomposites, their shape formed with self-assembly and their composition tuned with conversion reactions. (Image: AMOLF)
|
|
Finally, the team explored the potential applications of this new approach. For instance, they discovered that the nickel-containing nanocomposites can be used as catalysts for the dry-reforming process, which outperforms traditional catalysts at low temperatures.
|
|
Furthermore, the team synthesized shape-controlled magnetite (Fe3O4) nanocomposites that can be moved and reorientated using their magnetic properties. Finally, they created e-beam activated microscopic actuators by utilizing flexibility introduced during one of the ion exchange reactions in conjunction with the shrinking properties of the silica matrix.
|
|
In short, they discovered shape-preserving ion-exchange reactions that open up new routes towards self-assembled materials with various novel, functional properties.
|


