| Nov 04, 2022 |
New study introduces the best graphite films
(Nanowerk News) High-quality graphite has excellent mechanical strength, thermal stability, high flexibility and very high in-plane thermal and electric conductivities and, thus, is one of most important advanced materials for many applications, such as being used as the light thermal conductor of cell phones. For example, a specific type of graphite, Highly Ordered Pyrolytic Graphite (HOPG), is one of the mostly used lab materials.
|
|
These excellent properties originate from the layered structure of graphite, where the strong covalent binding between carbon atoms in a graphene layer contribute to the excellent mechanical properties, thermal and electric conductivities and the very weak interaction between graphene layers leads to the highly flexibility of graphite.
|
|
Although graphite has been discovered in nature for more 1000 years and its artificial synthesis has been explored for more than 100 years, the quality of graphite samples, either natural ones or synthesized ones, are far from ideal. Such as the size of the largest single crystalline graphite domains in graphitic materials are generally less than 1 mm, which is in sharp contrast to the size of many crystals, such as the size of quartz single crystal and silicon single crystals may reach meter scale.
|
|
The very small size of single crystalline graphite is due to the weak interaction between graphite layers, where the flatness of a graphene layer is hard to be maintained during the growth process and, thus, a graphite can be easily breaks into a few single crystals with disordered grain boundaries (See Figure 1).
|
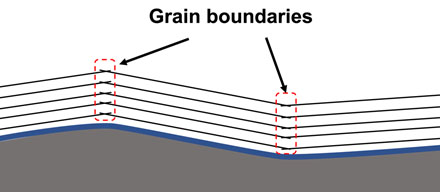 |
| Figure 1. Illustration of graphite growth on an rough substrate, on which disorders, namely grain boundaries, between single crystalline graphite islands nucleated on different flat terraces are inevitable. (Image: UNIST)
|
|
To solve the critical issue, Distinguished Professor of Ulsan National Institute of Science and Technology (UNIST) and his collaborators, Professor Kaihui Liu, Professor Enge Wang of Peking University, and others has proposed a strategy to synthesize single-crystalline graphite films orders of magnitude large, up to inch scale.
|
|
In their approach (Nature Nanotechnology, "Continuous epitaxy of single-crystal graphite films by isothermal carbon diffusion through nickel"), single crystalline Ni foils are used as a substrate and caron atoms are supplied from the back side of the Ni foils through an “isothermal dissolution-diffusion-precipitation process” (See Figure 2).
|
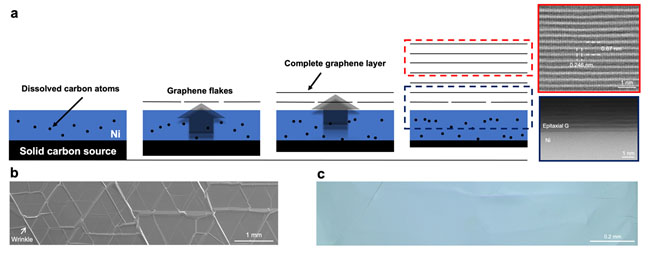 |
| Figure 2. (a) The mechanism of single crystalline graphite growth on a ultraflat single crystalline Ni foil: (i) carbon atoms of the solid carbon source dissolve into the single crystalline Ni; (ii) and the dissolved carbon atoms transport through the Ni foil to another side to forma graphene flakes; (iii) graphene flakes continuously merge into single crystalline graphene films and (iv) finally thick graphite films are formed on the single crystalline Ni substrate. (b) part of the experimentally grown inch-sized single crystalline graphite, on which the wrinkles formed during the cooling of the sample can be seen. (c) the ultra-flat graphite films formed by etching the substrate after growth. (Image: UNIST)
|
|
Instead of using gas phase carton source, they choose solid carbon materials to feed the graphite growth. Such a new strategy allows of ~1 inch single crystalline graphite films of 35 µm thick, or more than 100,000 graphene layers, within a few days. The single crystalline graphite has the recorded thermal conductivity of ∼2,880 Wm-1K-1, negligible impurity contents and smallest layer distances in compare with all available graphite samples.
|
|
“This success really on a few critical issues of the experimental design: (1) the successful synthesis of large size single crystalline Ni films serves as an ultra-flat substrate and thus the disorders in the synthesized graphite can be avoided; (2) the isothermal growth of 100,000 graphene layers over ~ 100 hours allows every graphene layer be synthesized under exact same chemical environment and temperature thus ensure the uniformity of the graphite quality; (3) continuous carbon feeding through the back side of the Ni foil allows the contiguous growth of graphene layers in a very large growth rate, ~ one layer per five seconds,” Professor Ding explained.
|


