| Nov 22, 2023 |
CRISPR-powered optothermal nanotweezers allow targeted manipulation of single DNA molecules
(Nanowerk News) For decades, researchers have sought ways to precisely manipulate and identify individual molecules like DNA in liquid environments. Such capabilities could revolutionize areas ranging from disease diagnosis to drug development. However, the randomness of molecular movements in fluids has hindered progress.
|
|
Now, scientists from Shenzhen University and the Chinese University of Hong Kong report promising advances in optical tweezing techniques that allow exquisite control over nanoscale biological particles (Light: Science & Applications, "CRISPR-powered optothermal nanotweezers: Diverse bio-nanoparticle manipulation and single nucleotide identification").
|
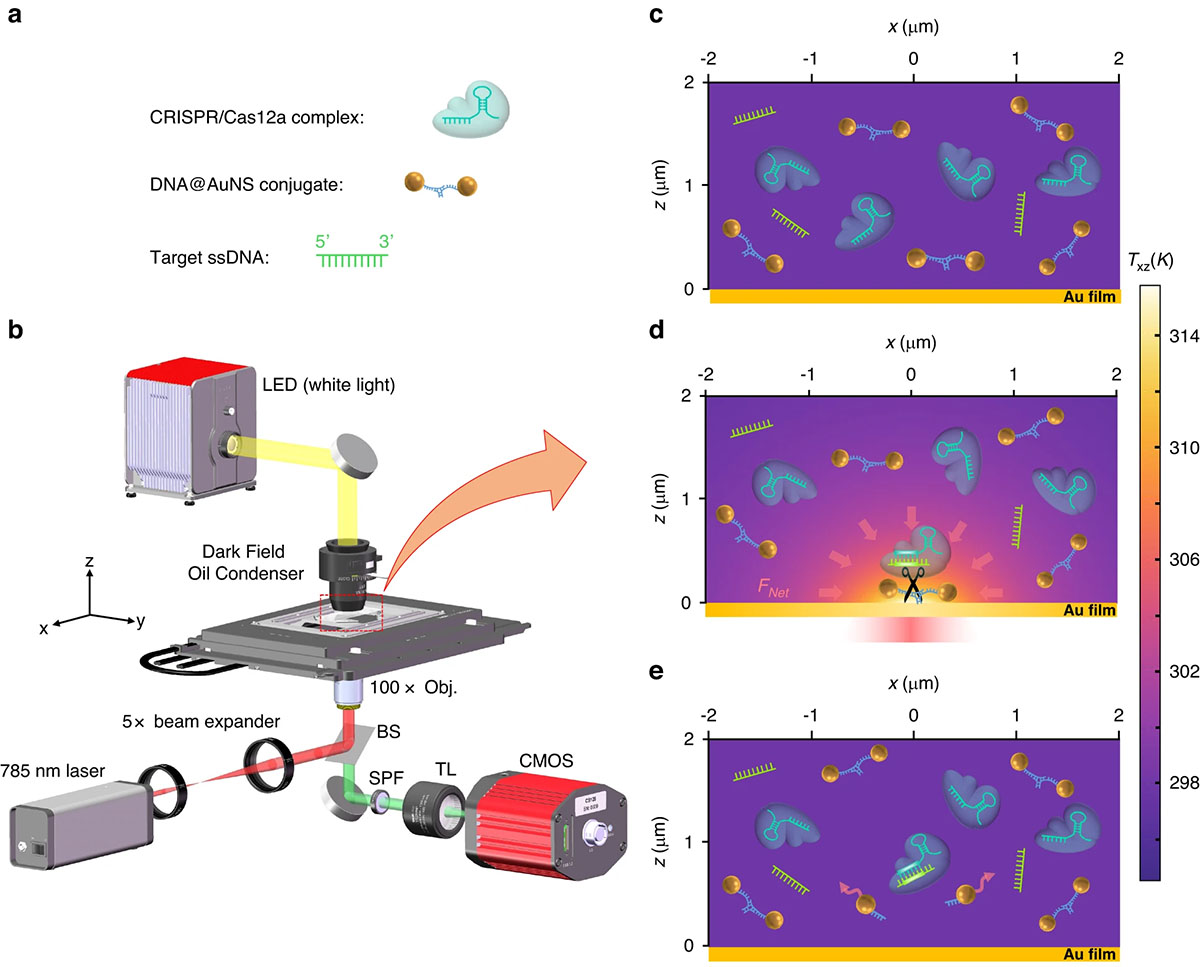 |
| a The diagrammatic sketch of the three components in the solution: DNA@AuNS conjugate, CRISPR/Cas12a complex, and target ssDNA. b Optical setup, the BS, SPF, and TL are beam splitter, short pass filter, and tube lens (f = 200 mm), respectively. Additional details of the setup are provided in the Materials and Methods section. c Dispersion of the three components in the solution without optical heating. d Optothermal net force induced migration and DNA@AuNS conjugate cleavage upon optical heating, the heating laser power is 0.5 mW. e Observation of the cleavage after the optical heating is switched off. (© Light: Science & Applications) (click on image to enlarge)
|
|
Optical traps, which use laser beams to capture microscopic objects, were themselves a transformative breakthrough awarded the 2018 Nobel Prize in Physics. But conventional tweezers have limitations that the new ‘CRISPR-powered optothermal nanotweezers’ (CRONT) aim to overcome – they struggle to trap nanoscale items like DNA and proteins. And crucially, classical traps cannot identify the molecular composition of captured particles without further probing.
|
|
The innovations of CRONT address these restrictions in two ways. First, they utilize thermodynamic forces like heat and concentration gradients to gather and manipulate biomolecules. Second, they incorporate CRISPR – the renowned DNA-cutting tool that earned the 2020 Nobel Prize -– to identify nucleotide sequences directly in the optical trap.
|
|
As lead author Han Zhang explains, “We designed CRONT to enrich target DNA molecules and meet the precise thermal requirements for CRISPR-mediated cleavage – all within an optical trap at the diffraction limit. This essentially turns optical tweezers into a versatile tool for manipulation and identification of single biomolecules.”
|
|
But why manipulate single molecules? Advances in this arena could drive rapid disease screening or precisely guide CRISPR to edit genes inside cells. Moreover, controlling nano-sized particles like DNA enables the study of biological mechanisms one molecule at a time. “But it’s incredibly difficult,” says Zhang. “The random shaking of molecules due to Brownian motion is proportionally more pronounced at smaller scales.”
|
|
Early optical traps utilized the momentum of light to overcome Brownian forces. But the required laser intensity often damaged delicate biomolecules. Later methods added things like plasmonics and electric fields. However, such tweezers still struggle with tiny biological targets like DNA. Moreover, optical traps alone cannot specify what molecule is captured – target verification requires secondary probes.
|
|
CRONT sidesteps these issues through thermodynamic forces induced by optothermal effects. A thin gold film heats up when illuminated by an infrared laser. In turn, the generated temperature field moves molecules via natural phenomena like thermophoresis and diffusiophoresis. “We meticulously analyzed how every force component gathers particles to the laser spot,” explains Zhang.
|
|
Remarkably, the researchers showed how varying just the illumination could accumulate diverse nanoscale entities like DNA, proteins, and nanoparticles to desired locations. Since the forces scale with an object’s size and surface chemistry, each biomolecule exhibited distinct trapping behavior. And unlike traditional optical traps, CRONT provided sufficient force to capture and manipulate single DNA strands.
|
|
But the most groundbreaking capability of CRONT is integrated target identification. The localized heat was tuned to 37 °C – the precise temperature for CRISPR-mediated DNA cleavage. With CRISPR elements introduced into the trapping region, sequences captured by CRONT can be directly interrogated and even snipped apart. By subsequently imaging the separated DNA-linked nanoparticles, the presence of target genes is confirmed.
|
|
This in situ approach attained incredible sensitivity down to just 25 attomoles per microliter – roughly 150 molecules. Even minute single nucleotide changes were identifiable. According to Zhang, “the specificity permits detecting viral mutations critical for emerging outbreaks like COVID-19”. No amplification or labeling is necessary, allowing rapid assessment.
|
|
CRONT uniquely merges optical manipulation, thermally controlled reactions, and CRISPR-based sensing in one integrated platform. The researchers suggest CRONT could advance point-of-care molecular diagnosis and next-generation genomic editing techniques regulated in space and time at single cell resolution. While still an initial proof of concept, CRONT demonstrates thermodynamic optical trapping can open unprecedented possibilities.
|

