| Mar 25, 2024 |
Filming ultrafast molecular motions in single crystal
(Nanowerk News) Understanding the behavior of matter is crucial for advancing scientific fields like biology, chemistry, and materials science. X-ray crystallography has been instrumental in this pursuit, allowing scientists to determine molecular structures with precision. In traditional X-ray crystallography experiments, a single crystal is exposed to X-rays multiple times to obtain diffraction signals. This poses a problem, where the sample has its structure altered or damaged by X-ray exposure.
|
|
In recent years, advances in technology have allowed for the development of “time-resolved serial femtosecond crystallography” (TR-SFX). In serial crystallography, a crystal is exposed to X-rays only once, which allows for the measurement of the sample in the best possible state where the crystal is not damaged by X-rays. This is then combined with the popular time-resolved technique, which allows the structural changes of molecules in crystals to be followed in real time during a reaction.
|
|
However, TR-SFX so far has only been limited to the study of protein samples. If the usage of TR-SFX can be extended to non-protein samples, it will unlock opportunities to investigate real-time motion across a wider range of materials, encompassing those crucial for semiconductors and batteries.
|
|
For the first time, researchers led by Director IHEE Hyotcherl of the Center for Advanced Reaction Dynamics within the Institute for Basic Science (IBS) have applied TR-SFX to a system other than proteins. The material they chose was a sample called porous coordination network–224(Fe), PCN–224(Fe), to demonstrate the feasibility of serial crystallography at the molecular level, allowing them to observe molecular motion in real time with atomic resolution. The sample consists of carbon monoxide (CO) adsorbed onto iron porphyrin (Fe porphyrin) derivatives and zirconium (Zr) clusters repeated in a metal–organic framework.
|
|
The findings have been published in Nature Chemistry ("Dynamic 3D structures of a metal–organic framework captured with femtosecond serial crystallography").
|
|
The reason why TR-SFX was previously limited to only studying protein samples was because much higher standards are required for evaluating the structures of non-protein samples. Hence, the IBS team had to greatly improve the specification of the crystallography in order to meet these high criteria. The team’s setup revealed the crystal structure at a total of 33 time points ranging from 100 femtoseconds to 3 nanoseconds (10-9 seconds).
|
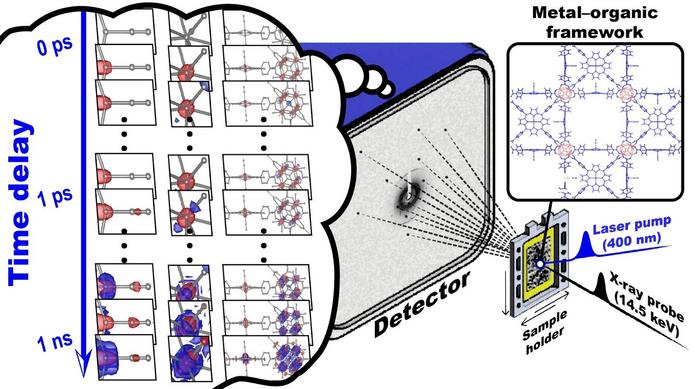 |
| Scheme of a time-resolved serial femtosecond crystallography (TR-SFX) experiment on porous coordination network–224(Fe), PCN–224(Fe). The crystals of PCN–224(Fe) are exposed to an intense femtosecond UV laser pulse to trigger a reaction. Ultrafast structural changes of iron porphyrin and zirconium clusters in PCN–224(Fe) were directly visualized using X-ray pulses from the X-ray free-electron laser facility with femtosecond and angstrom spatio-temporal resolution. By measuring the X-ray diffraction patterns produced by the X-ray pulses over time, the molecular structure of PCN–224(Fe) after the reaction was observed. (Image: Institute for Basic Science)
|
|
This is an advance over previous TR-SFX studies of the proteins, which typically report crystal structures at only about 10 time points. This substantial increase in temporal resolution, nearly three times greater than previous studies on proteins, allowed for a more accurate representation of structural changes over a long period of time.
|
|
When PCN–224(Fe) is irradiated with light, the CO adsorbed on the Fe porphyrin is dissociated, initiating a cascade of structural changes. Using the improved TR-SFX, researchers were able to observe these structural changes with unprecedented detail - with a femtosecond time resolution of 10-15 seconds and an atomic resolution of 10-10 meters (or angstroms).
|
|
They were able to identify three different pathways of structural change: doming, the movement of iron atoms in iron porphyrins out of the porphyrin plane; phonon mode of zirconium and iron atoms; and random vibrational motion with increasing temperature. With this study, the researchers have shown that it is possible to apply TR-SFX measurements to chemical systems, an important step forward in demonstrating the practicality of the technique.
|
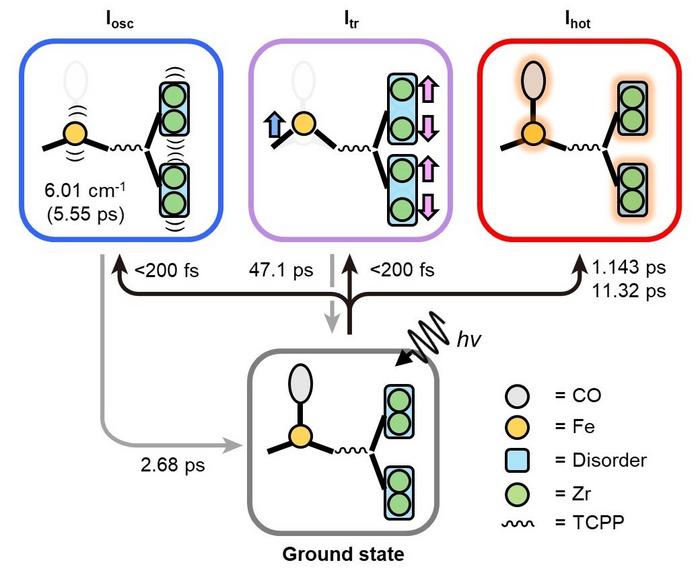 |
| Photoinduced trifurcating structural changes of PCN–224(Fe) observed by means of TR-SFX. Upon irradiation, one CO molecule dissociates from iron porphyrin and organized motions occur within 200 fs. The organized motions include the oscillatory motion (Iosc) and the formation of the transient structure (Itr). Furthermore, alongside the organized motions, the thermally hot structure (Ihot) emerges through random atomic movements. (Image: Institute for Basic Science)
|
|
The study marks a major milestone for the scientific community as it is the first time molecular behavior has been observed in real-time using serial crystallography. By using TR-SFX, a technique that provides high spatiotemporal resolution, the team was able to capture minute structural changes in solid-state molecules in real-time.
|
|
Director Ihee of the Center for Advanced Molecular Reaction Dynamics said, “Since the technical advances and analytical methods proposed in this study can be widely used to observe many other crystalline phase reactions of various molecular systems, this research not only opens new horizons in the field of molecular structure research but also has endless applications in future scientific discoveries.”
|


