| Posted: Jun 23, 2017 | |
Nanostructured microfluidics platform detects infectious pathogens(Nanowerk News) In a recent paper in Small ("A Nanostructured Microfluidic Immunoassay Platform for Highly Sensitive Infectious Pathogen Detection"), researchers from The Pennsylvania State University report a new zinc oxide (ZnO) nanorods integrated microchip (ZnO-NIM) that captures avian influenza virus (AIV) on immunologically functionalized ZnO nanorod surface and detect viruses by multiplexed sandwich immunoassay. |
|
 |
|
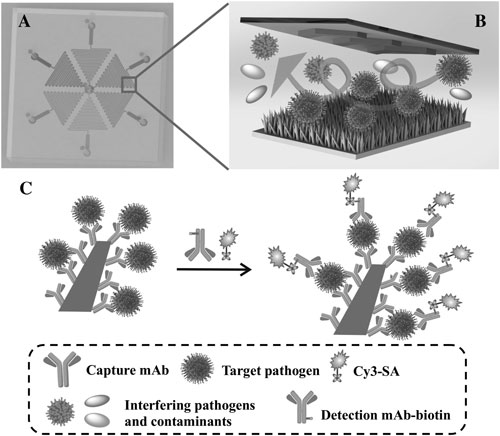
| Principle of the multiplexed pathogen capture and detection on the ZnO-NIM (not drawn to scale). A) Top view of a 3D illustration showing the layout of the microfluidic chip. The six branched microchannels are arranged in hexagon and can be loaded with six different samples (highlighted as different colors), respectively. B) Illustration showing the process of the pathogen capture on the 3D nanostructured ZnO surface conjugated with capture mAb on the ZnO surface inside the ZnO-NIM. The ceiling of the microfluidic channels contains herringbone-shaped structures to enhance mixing. C) Illustration of the sandwich immunoassay for pathogen detection on a single ZnO nanorod inside the ZnO-NIM. Biotin-labeled detection monoclonal antibodies (mAb-biotin) bind to pathogens. Fluorescence signal is produced by Cy3-SA. (© Wiley-VCH Verlag) (click on image to enlarge) | |
| The authors point out that they synthesized the ZnO nanorods on the activated glass slides in a low-cost and scalable batch process that does not depend on cleanroom facilities. | |
| To improve the interaction on the ZnO nanorod surface, herringbone structures were incorporated inside the microfluidic device for enhanced mixing. | |
| The team demonstrated highly selective detection of H5N2 subtype AIV by fast continuous flow in the microfluidic channels that effectively minimizes nonspecific adsorption. | |
| The entire detection process could be completed within 1.5 hours, while the limit of detection (LOD) for the H5N2 AIV was measured as 3.6 × 103 EID50 mL-1. | |
| This platform shows the capability for simultaneous detection of multiple viral pathogens by spatial encoding different antibodies onto the same ZnO-NIM. | |
| Moreover, the scientists further demonstrated the captured H5N2 AIV could be released by simply dissolving the ZnO nanorods in acidic environment and the released H5N2 AIV could be detected by the real-time RT-PCR (rRT-PCR). | |
| The successful virus release enables subsequent off-chip analyses of the captured viruses by ZnO-NIM, which complements to the on-chip multiplexed sandwich immunoassay and greatly expands its capability. | |
| As the authors note, this sensitive, selective, multiplex portable technology and the integrated fluorescence sandwich immunoassay can be expanded to detect other pathogens and disease biomarkers. |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
