| Posted: Aug 26, 2017 | |
Cascade amplification release nanoparticle to overcome tumor drug resistance(Nanowerk News) Researchers have developed a cascade amplification release nanoparticle (CARN) by simultaneously loading a high drug content of Lapa and an ROS-responsive doxorubicin (DOX) prodrug (BDOX) in a novel block copolymer, poly(ethylene glycol)–poly[2-(methylacryloyl)ethylnicotinate] (PEG–PMAN), micelle. |
|
| The team reported their findings in Advanced Materials ("A Tumor-Specific Cascade Amplification Drug Release Nanoparticle for Overcoming Multidrug Resistance in Cancers"). | |
 |
|
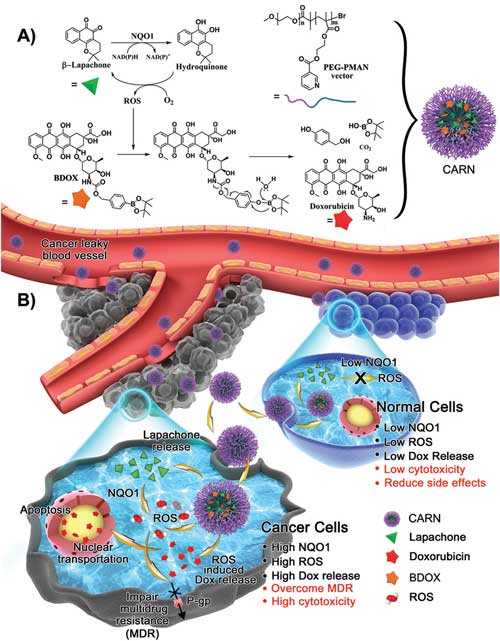
| Schematic illustration of a cascade amplification release nanoparticle (CARN) and its sequential release process. A) Chemical structure of β-lapachone, the prodrug BDOX, and the poly(ethylene glycol)–poly[2(methylacryloyl)ethylnicotinate] (PEG–PMAN) carrier and their cascade drug release mechanism. B) After intravenous injection, CARNs are accumulated in tumor tissues because of the EPR effect and internalized by cancer cells. Lapa is first discharged from the nanoparticle and generates abundant ROS via the catalysis of the overexpressed NQO1 enzyme, which feeds back to the nanoparticle to transform BDOX into DOX and promotes substantially accelerated drug release. Meanwhile, the generated ROS can also inhibit the function of P-gp, block the efflux of DOX, facilitate the nuclear transportation of DOX, and finally induce apoptosis. In normal cells, the intracellular ROS level is barely affected by Lapa due to their low NQO1 expression, which keeps BDOX inert and innocuous in the nanoparticle. (© Wiley) (click on image to enlarge) | |
| The low toxicity and high hydrophobicity of ROS-responsive BDOX, which is derived from doxorubicin by incorporating a boronate moiety, could reduce its toxicity to normal cells and improve its stability in the nanoparticles, thus avoiding undesired extracellular release until ROS activation. | |
| After endocytosis by cancer cells, the CARNs would first release Lapa, which would then produce a remarkable increase in the ROS level via NQO1 (NAD(P)H:quinone oxidoreductase-1) catalysis. | |
| The generated ROS would subsequently activate the BDOX prodrug, leading to a sequential and amplified drug release process. | |
| More importantly, as the scientists point out, Lapa could synergize with DOX and dramatically reverse drug resistance in cancer cells by preventing drug efflux and promoting nuclear transportation. | |
| Moreover, Lapa had relatively low cytotoxicity and induced an insignificant ROS increase in normal cells because of their low NQO1 expression, interrupting the cascade DOX release process and resulting in a low cytotoxicity of the CARNs against normal cells. | |
| Integrating multiple mechanisms into a single CARN would reduce the side effects of the encapsulated anticancer drug, reverse drug resistance, and substantially enhance therapeutic efficacy. | |
| "With its high and selective cytotoxicity against MDR cancer cells and improved pharmacokinetics, CARN remarkably enhanced the in vivo antitumor efficacy against MCF-7 ADR xenograft tumors with an IRT of 92.8%, which was much higher than of DOX (50.3%) or P-Lapa (64.4%), and decreased the side effects of DOX and Lapa as well," the authors conclude their report. "This cascade amplification drug release system has provided a new therapeutic strategy for developing DDSs with high selectivity for cancer cells and the capability to overcome MDR in cancers." |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
