| Posted: Nov 03, 2017 | |
New work advances the control of chemical reactions at the nanoscale(Nanowerk News) By combining gold nanohole arrays and light irradiation, silver nanoparticles and polypyrrole can selectively be synthesized in the resonant area, and the distributions of chemical products and surface plasmon energy are well matched. These results have been reported in ACS Nano ("Plasmonic Nanochemistry Based on Nanohole Array"). |
|
| The authors investigated the plasmonic performance of a gold nanohole array film in the synthesis of silver nanoparticles and of polypyrrole. They found that silver nanoparticles grow exclusively in nanoholes and form a ring shape with a distribution perfectly matching that of the enhanced electric field. | |
| The researchers demonstrated the generality of the approach by extending the reaction to polypyrrole synthesis in nanoholes. Overall, the results reported in this work will advance the control of chemical reactions at the nanoscale and will inspire a facile way to pursue patterned chemical reactions. | |
 |
|
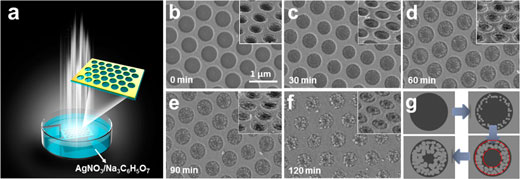
| (a) Experimental setup: a glass substrate coated with the gold nanoarray film is immersed into the solution and irradiated by a white LED lamp. Scanning electron microscopy (SEM) images of the gold nanoarrays with silver nanoparticles selectively growing in the interior of the holes with t = (b) 0 min, (c) 30 min, (d) 60 min, (e) 90 min, and (f) 120 min. The hole diameter is 500 nm. The insets show the 45° tilting SEM images of the corresponding samples. The scale bar in (b) corresponds to 1 µm and applies to all the SEM images. (g) Schematics showing the reaction process. t increases as the arrows point. The red dotted lines indicate the ring-shape distribution of the silver nanoparticles. (© ACS) (click on image to enlarge) | |
| Previous work has demonstrated the great potential of using plasmonics to spatially control chemical reactions, this research field is only in its infancy as they do not make use of the large variety of plasmonic structures and chemical reactions. Most efforts are currently put into investigating the effect of metallic nanoparticles, especially coated with TiO2, on chemical reactions. | |
| This demonstrates the key function of the plasmonic field for the site-selective chemical reaction. Compared with nanoparticle-based plasmonic chemistry, film-based plasmonic chemistry shows better spatial control to achieve ordered arrays of chemical products and may make a contribution in fabricating chemical patterns. | |
| "Moreover, because the growth of silver nanoparticles follows the distribution of the enhanced electric field, more chemical patterns can be fabricated by controlling the electric field distribution based on different incident light and plasmonic structures," the authors conclude. "The combination of plasmonics and chemical reactions is promising to inspire another progressing way for both traditional chemistry and nanofabrication techniques." |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
