| Posted: Jan 15, 2018 | |
Scalable synthesis of nanoporous metal structures(Nanowerk News) A new study published in ACS Nano ("A Scalable Synthesis Pathway to Nanoporous Metal Structures") details a simple, versatile, scalable synthesis route to nanoporous metals (NPMs) via solid-state conversion reactions. |
|
| Unlike the dealloying process, the oldest and most widely researched synthesis route for NPMs where the porous structure forms simultaneously while removing the less-noble element of an atomically mixed alloy, this method first fashions a nanocomposite of the desired pure metal with an ionic compound that can be removed by dissolution with a common organic solvent. | |
 |
|
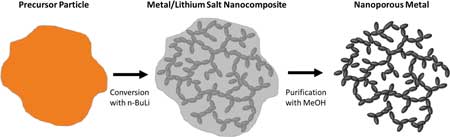
| Illustration of NPM preparation via conversion reaction synthesis. An anhydrous transition-metal halide precursor (typically a chloride compound in this study) is reacted with n-BuLi and converted to a metal/lithium halide nanocomposite. The lithium halide is removed by dissolving it with methanol, leaving behind a NPM. (© ACS) | |
| With conversion reaction synthesis the nanoporous metal formation occurs in two phases. The nanoporous structure originally forms in the nanocomposites as the metal and lithium halide phases spinodally decompose as they are produced, with the metal atoms being thermodynamically driven together into a network of fine filaments surrounded by the corresponding lithium halide. | |
| The dimensions of the metal network are characteristic of the target metal atoms’ mobility and the amount of lithium halide produced by the reaction between the precursor and the organolithium reagent. The lithium halide provides a kinetic barrier to excess metal atom agglomeration, which locks the metal network into a metastable state. | |
| Then, removing the lithium with methanol reveals the porous network, but the purification process causes reconstruction that decreases the final pore volume. | |
| The authors from the University of California, San Diego, note that conversion synthesis of nanoporous metals is qualitatively similar to dealloying – and can produce similar structures – and other selective etching processes employed in nanomaterials synthesis, such as the techniques used to make MXenes. | |
| If one uses each respective method to prepare nanoporous gold, both will produce similar ligamented networks with very similar specific surface area and pore volume. | |
| While the application of the dealloying method is determined by the availability of the alloy precursor and the removal of the reactive element, conversion synthesis is compatible with any metal halide precursor with an appropriate electrochemical potential, which allows the synthesis of pure nanoporous metal structures of a variety of transition metals. | |
| "We are also able to synthesize mixed nanoporous metals from mixtures of precursors, which in some cases exhibit finer nanostructures and superior surface properties when compared to nanoporous structures of the constituent metals," the authors conclude their report. "Mixing precursors opens a large parameter space for further design and engineering of nanoporous metals and further proves the versatility of this synthesis method. With the abundance of compatible precursor candidates and with the simplicity and scalability of these methods, conversion synthesis provides a wide and accessible design space for the development of nanoporous metal technology." |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
